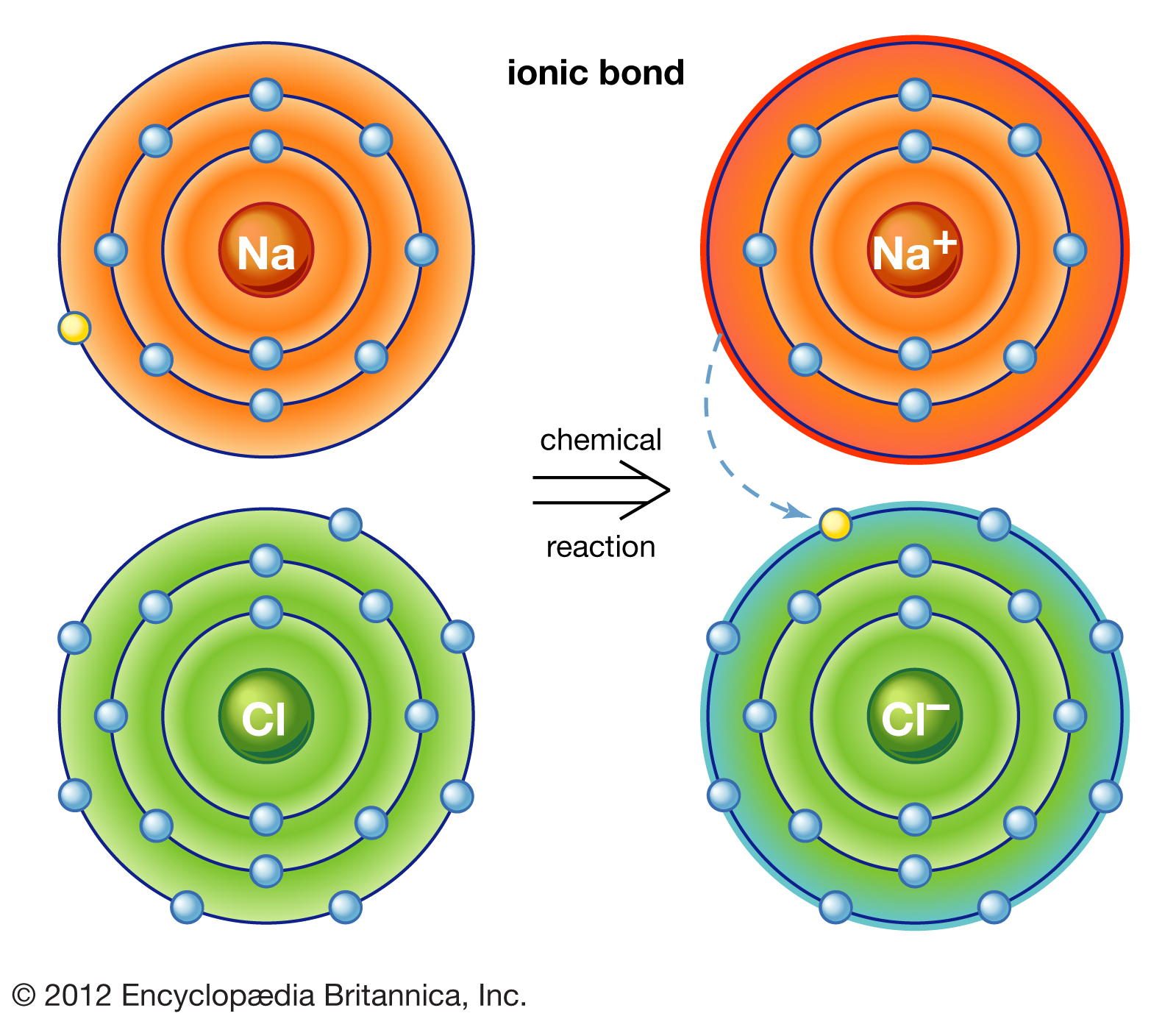
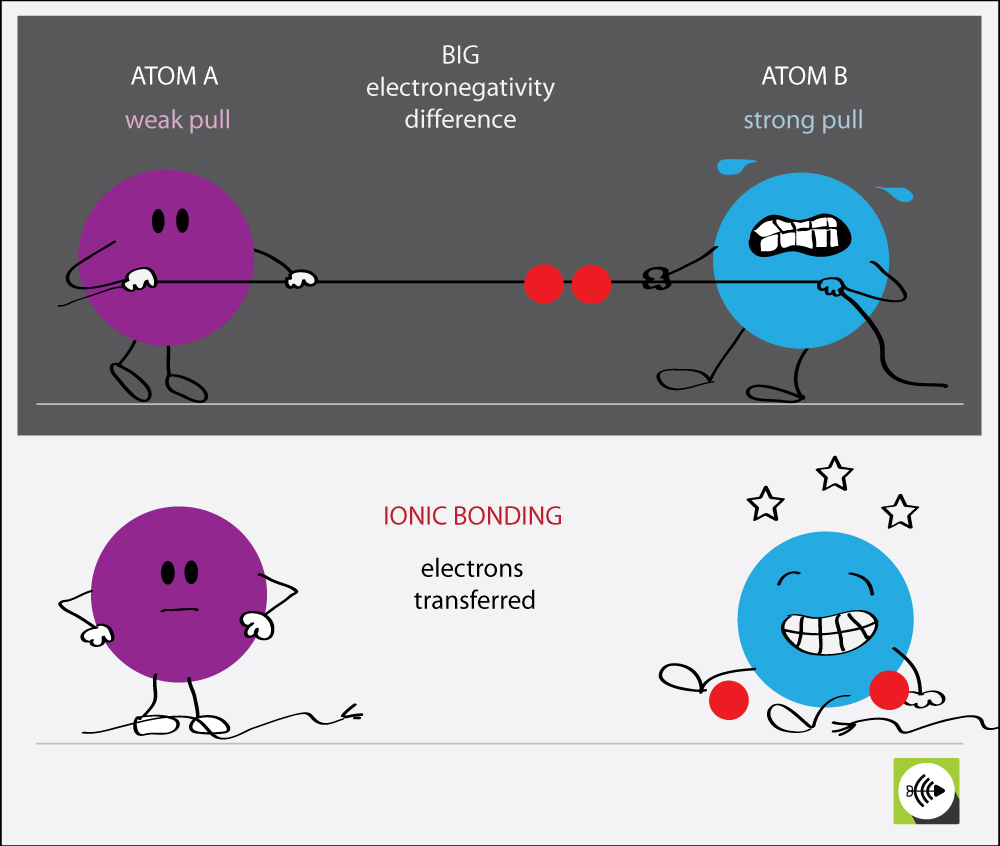
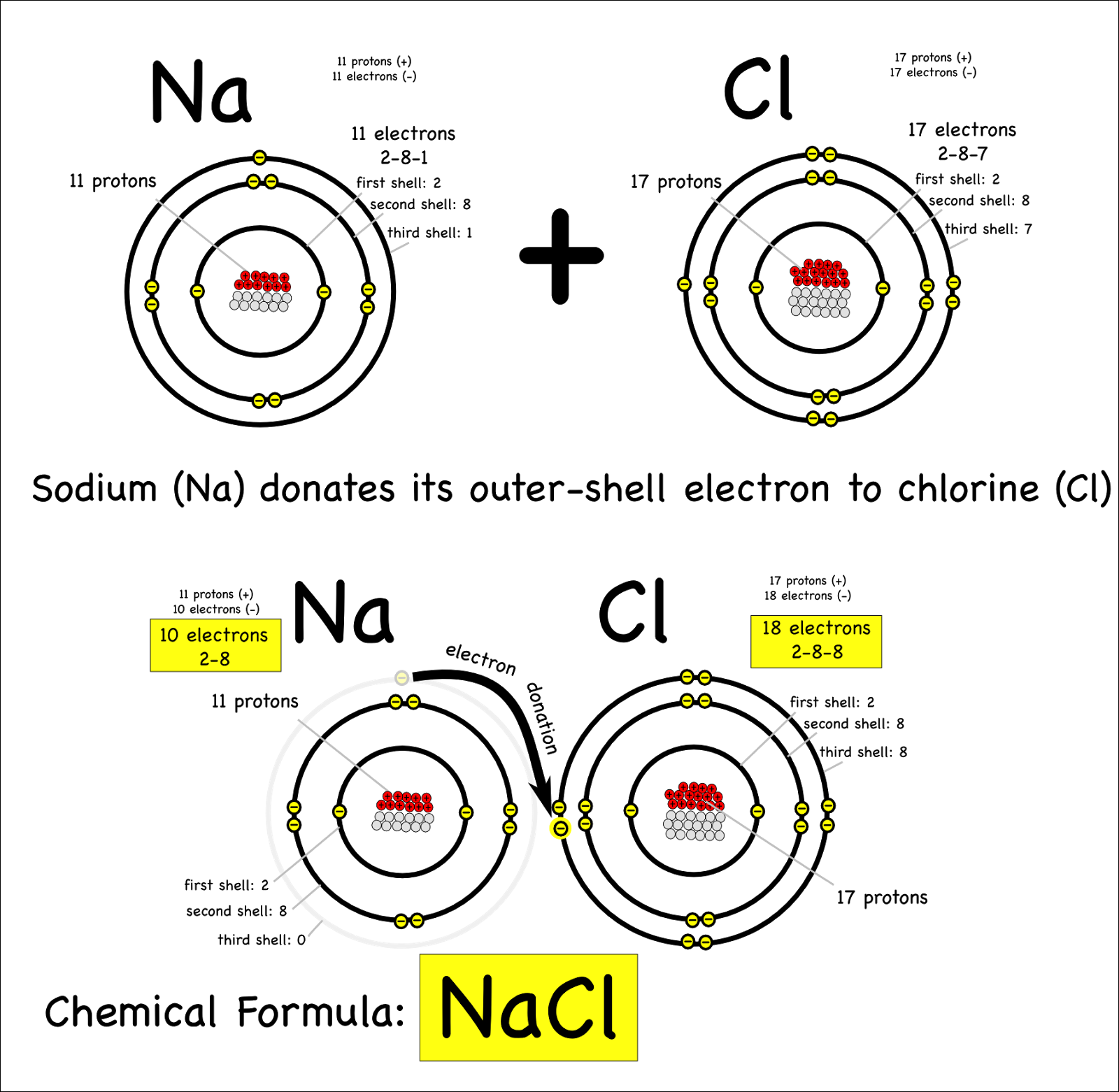
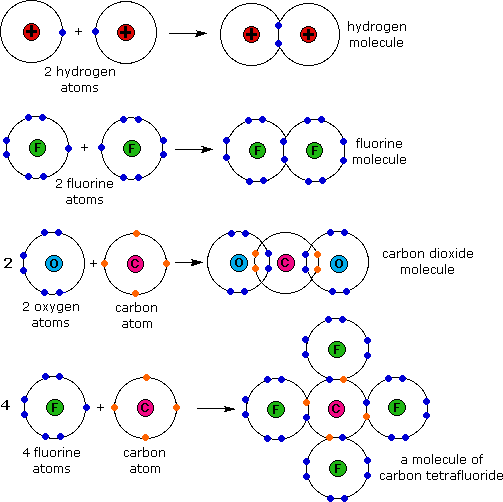
Why Do Atoms Form Ionic And Covalent Bonds - Ionic bonds require at least one electron donor and one electron acceptor. The bond made by electron sharing is called a covalent bond. They differ in their structure and properties. Despite our focus on the octet rule, we must remember that for small atoms, such as. In ionic bonding, atoms transfer electrons to each other.
Despite our focus on the octet rule, we must remember that for small atoms, such as. They differ in their structure and properties. In ionic bonding, atoms transfer electrons to each other. The bond made by electron sharing is called a covalent bond. Ionic bonds require at least one electron donor and one electron acceptor.
In ionic bonding, atoms transfer electrons to each other. Despite our focus on the octet rule, we must remember that for small atoms, such as. They differ in their structure and properties. The bond made by electron sharing is called a covalent bond. Ionic bonds require at least one electron donor and one electron acceptor.
What Best Describes a Covalent Bond KenziehasNicholson
In ionic bonding, atoms transfer electrons to each other. Ionic bonds require at least one electron donor and one electron acceptor. The bond made by electron sharing is called a covalent bond. They differ in their structure and properties. Despite our focus on the octet rule, we must remember that for small atoms, such as.
chemistry knowledge Comparison between Covalent and Ionic Bond
Ionic bonds require at least one electron donor and one electron acceptor. The bond made by electron sharing is called a covalent bond. Despite our focus on the octet rule, we must remember that for small atoms, such as. In ionic bonding, atoms transfer electrons to each other. They differ in their structure and properties.
Why do atoms form ionic bonds? YouTube
The bond made by electron sharing is called a covalent bond. Despite our focus on the octet rule, we must remember that for small atoms, such as. In ionic bonding, atoms transfer electrons to each other. Ionic bonds require at least one electron donor and one electron acceptor. They differ in their structure and properties.
Ionic Bond And Covalent Bond Venn Diagram Amashusho Images
Despite our focus on the octet rule, we must remember that for small atoms, such as. In ionic bonding, atoms transfer electrons to each other. Ionic bonds require at least one electron donor and one electron acceptor. They differ in their structure and properties. The bond made by electron sharing is called a covalent bond.
Electronegativity Bond Scale Surfguppy Chemistry made easy for
The bond made by electron sharing is called a covalent bond. In ionic bonding, atoms transfer electrons to each other. They differ in their structure and properties. Ionic bonds require at least one electron donor and one electron acceptor. Despite our focus on the octet rule, we must remember that for small atoms, such as.
Fourth Grade GC August 2013
Despite our focus on the octet rule, we must remember that for small atoms, such as. In ionic bonding, atoms transfer electrons to each other. The bond made by electron sharing is called a covalent bond. They differ in their structure and properties. Ionic bonds require at least one electron donor and one electron acceptor.
What Happens When Two Nitrogen Atoms Share Electrons MarisolkruwLee
Despite our focus on the octet rule, we must remember that for small atoms, such as. They differ in their structure and properties. The bond made by electron sharing is called a covalent bond. In ionic bonding, atoms transfer electrons to each other. Ionic bonds require at least one electron donor and one electron acceptor.
Ionic Bonding Diagram For Lithium Fluoride
They differ in their structure and properties. The bond made by electron sharing is called a covalent bond. Despite our focus on the octet rule, we must remember that for small atoms, such as. In ionic bonding, atoms transfer electrons to each other. Ionic bonds require at least one electron donor and one electron acceptor.
Ionic bonding Wikipedia
The bond made by electron sharing is called a covalent bond. In ionic bonding, atoms transfer electrons to each other. Despite our focus on the octet rule, we must remember that for small atoms, such as. Ionic bonds require at least one electron donor and one electron acceptor. They differ in their structure and properties.
Expanded Electron Configuration of Chlorine Womack Thille
Ionic bonds require at least one electron donor and one electron acceptor. In ionic bonding, atoms transfer electrons to each other. They differ in their structure and properties. Despite our focus on the octet rule, we must remember that for small atoms, such as. The bond made by electron sharing is called a covalent bond.
They Differ In Their Structure And Properties.
The bond made by electron sharing is called a covalent bond. Ionic bonds require at least one electron donor and one electron acceptor. In ionic bonding, atoms transfer electrons to each other. Despite our focus on the octet rule, we must remember that for small atoms, such as.