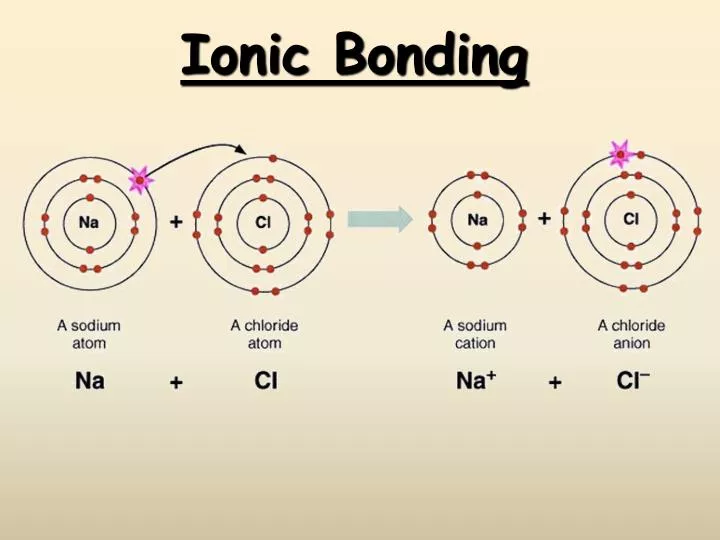
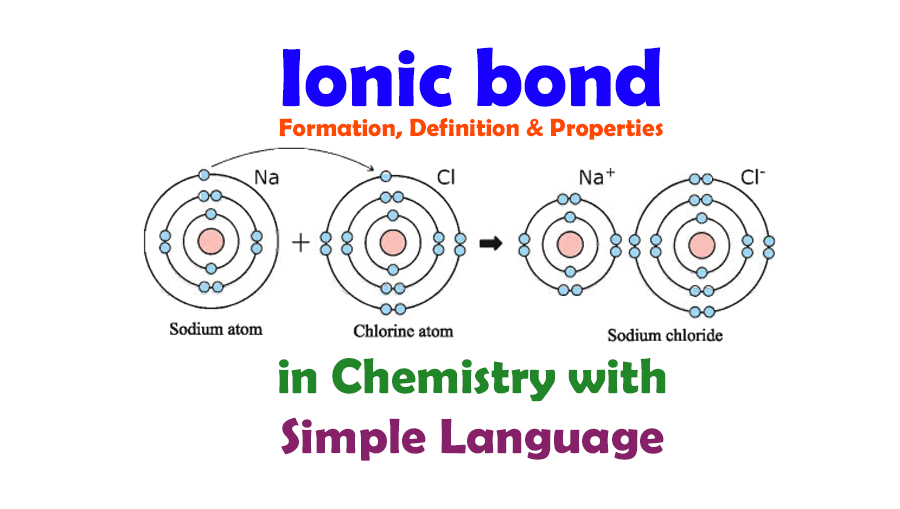
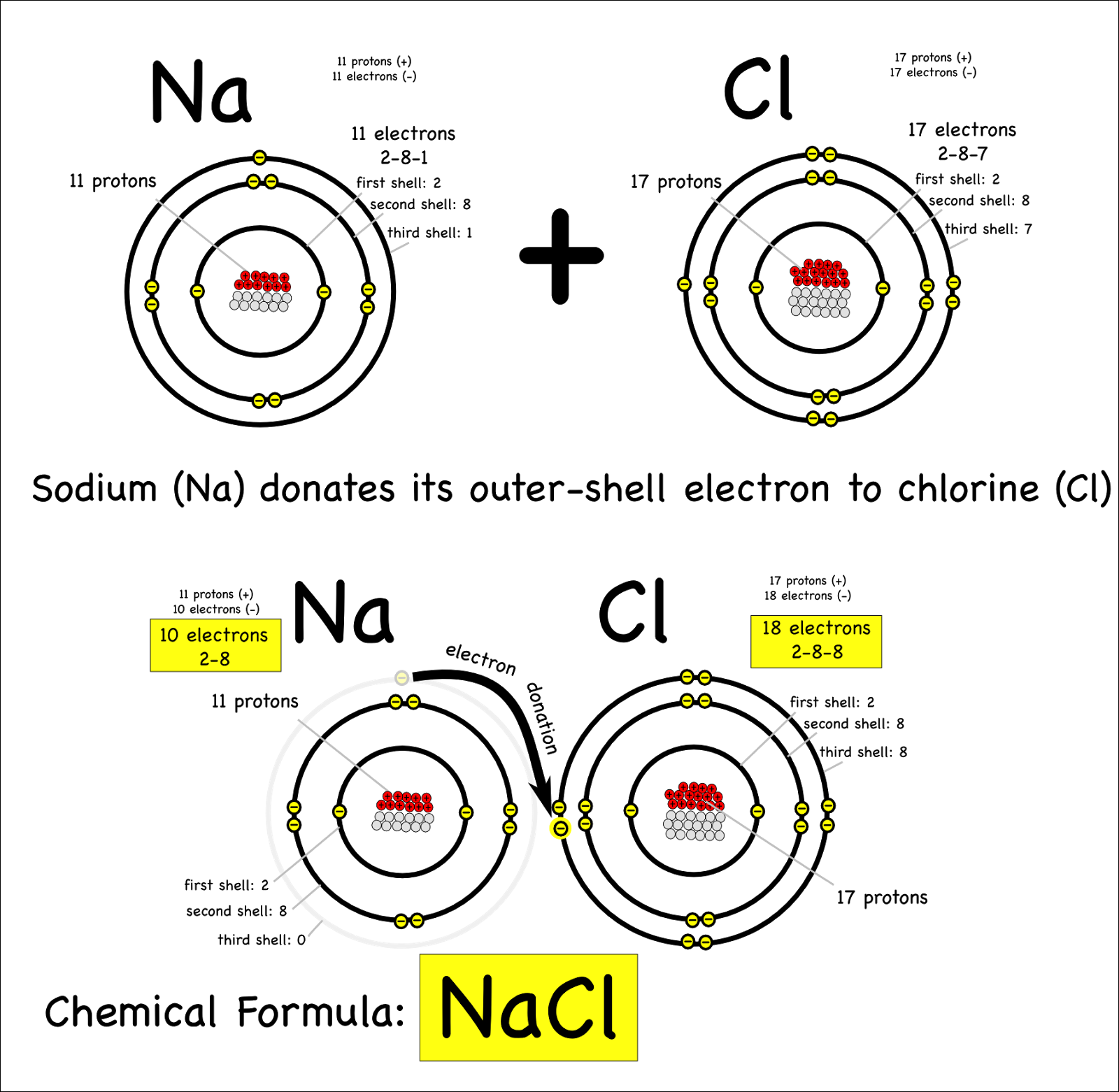
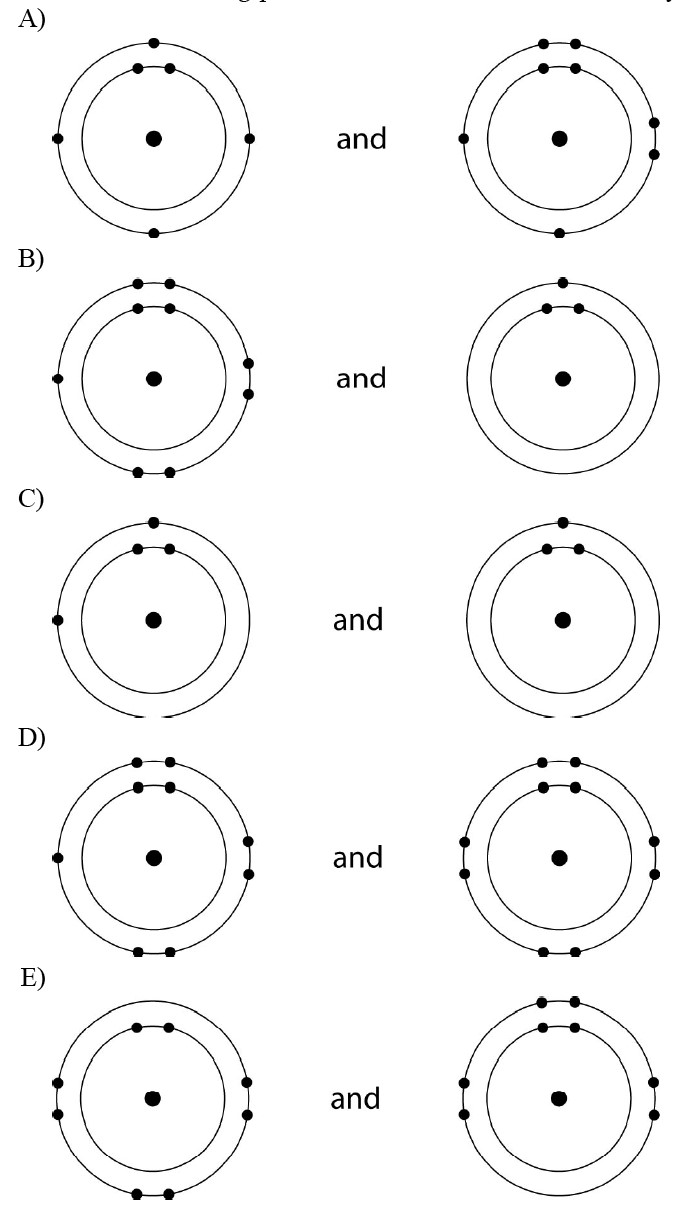
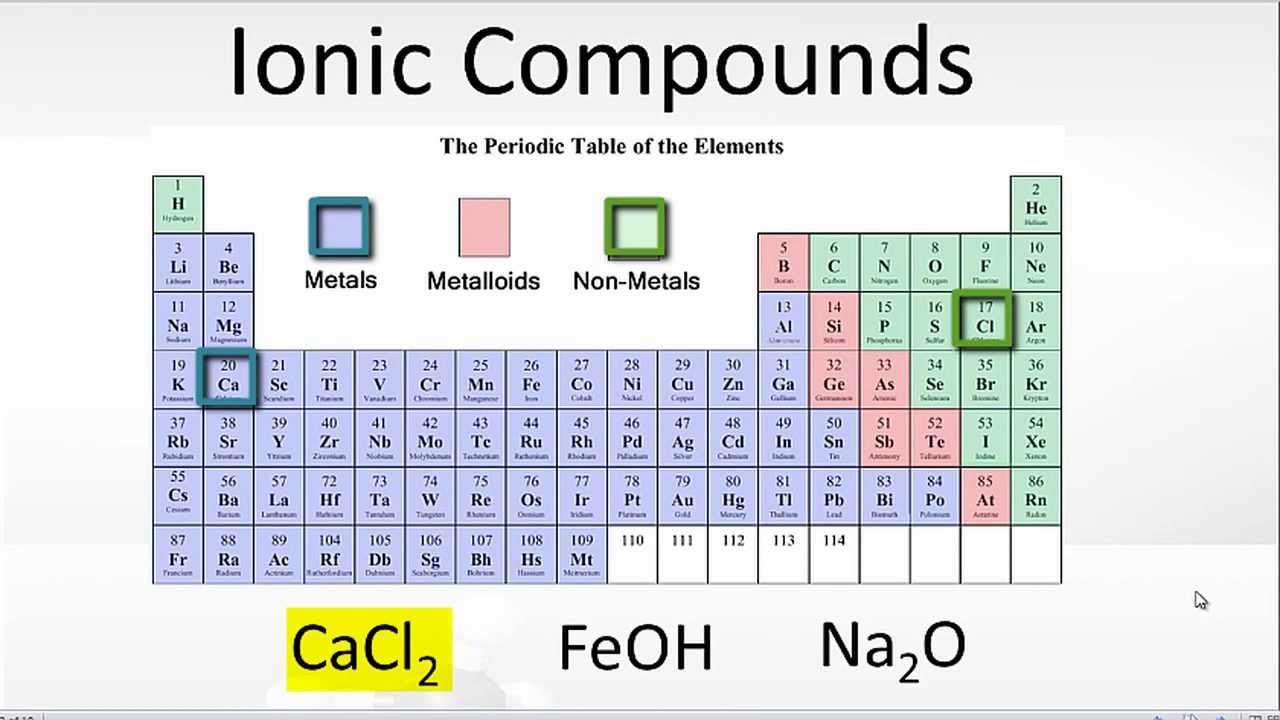
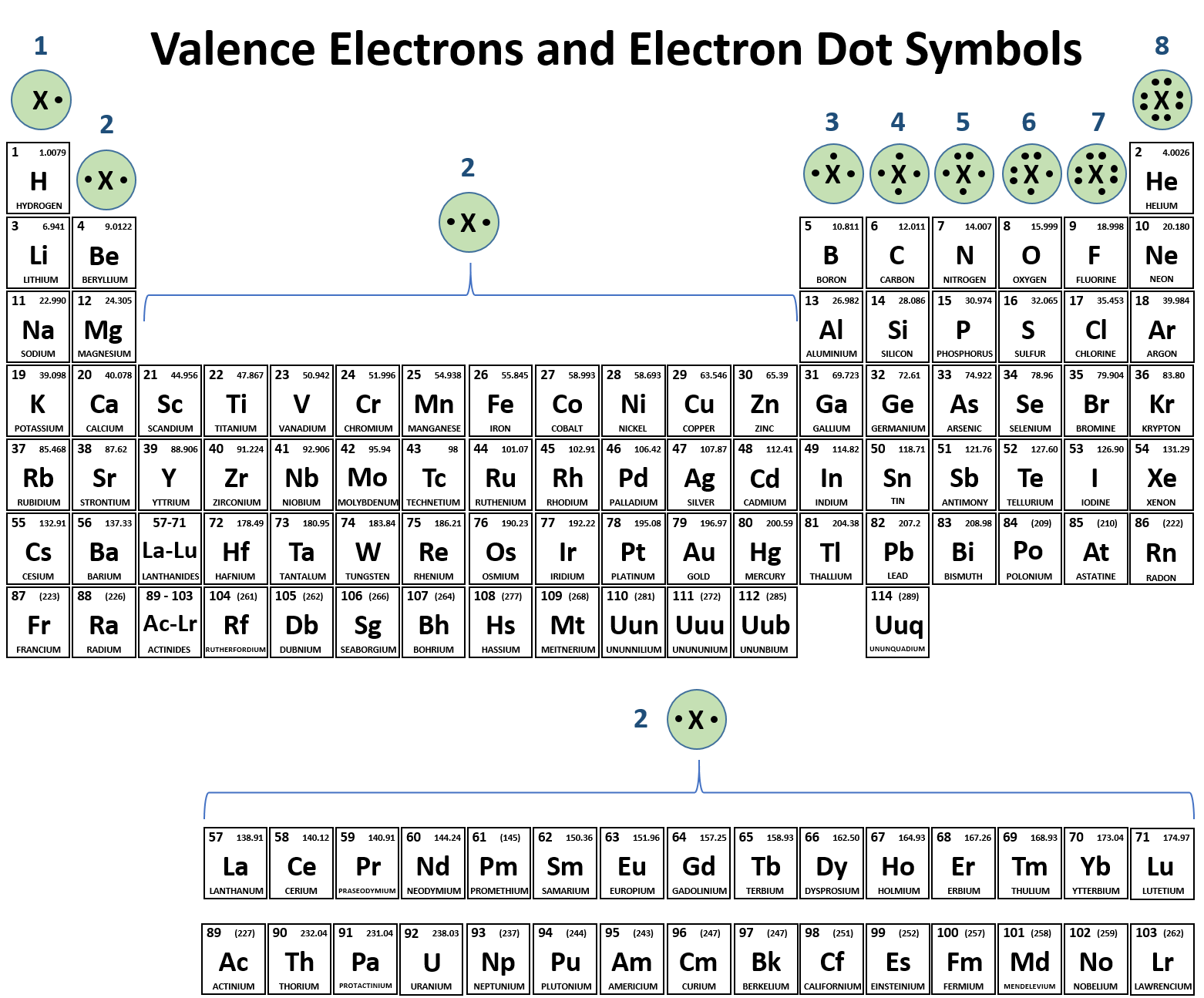
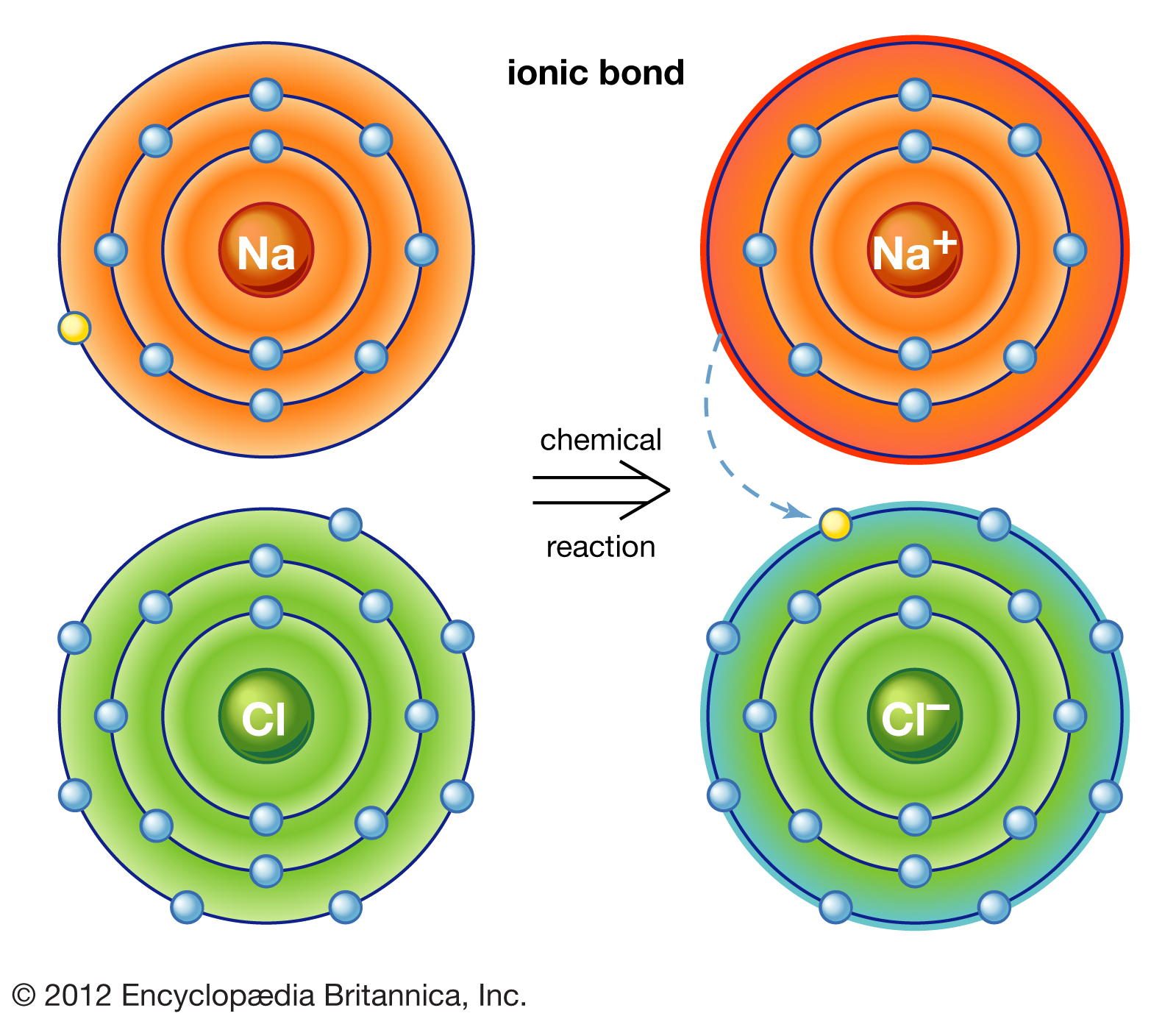
Which Pair Of Elements Would Form An Ionic Bond - A metal (which forms the cations) and a nonmetal (which forms the anions). In modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element) were transfered between the outer rings. It is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a. Which pair of elements will form an ionic bond? Binary ionic compounds are composed of just two elements: Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Therefore, they will form ionic bond.
It is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: In modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element) were transfered between the outer rings. Which pair of elements will form an ionic bond? Therefore, they will form ionic bond. Binary ionic compounds are composed of just two elements: A metal (which forms the cations) and a nonmetal (which forms the anions).
Binary ionic compounds are composed of just two elements: Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: It is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a. A metal (which forms the cations) and a nonmetal (which forms the anions). Therefore, they will form ionic bond. Which pair of elements will form an ionic bond? In modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element) were transfered between the outer rings.
PPT Ionic Bonding PowerPoint Presentation, free download ID2683450
It is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a. Which pair of elements will form an ionic bond? In modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element) were transfered between the outer rings. Compounds composed of ions are called ionic.
Ionic Bond and Ionic Bond Formation, Definition, Properties in
Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: It is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a. Binary ionic compounds are composed of just two elements: In modern language, the central idea of an ionic bond is that electrons.
[Solved] Which pair of elements will form an ionic compound? Multiple
In modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element) were transfered between the outer rings. A metal (which forms the cations) and a nonmetal (which forms the anions). Which pair of elements will form an ionic bond? It is composed of one phosphorus atom and four oxygen atoms covalently.
Fourth Grade GC August 2013
A metal (which forms the cations) and a nonmetal (which forms the anions). Which pair of elements will form an ionic bond? Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Therefore, they will form ionic bond. It is composed of one phosphorus atom and four oxygen atoms covalently.
Answered Consider the following pairs of atoms.… bartleby
Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Which pair of elements will form an ionic bond? Binary ionic compounds are composed of just two elements: Therefore, they will form ionic bond. A metal (which forms the cations) and a nonmetal (which forms the anions).
Examples of Ionic Bonding YouTube
Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: A metal (which forms the cations) and a nonmetal (which forms the anions). Binary ionic compounds are composed of just two elements: It is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a..
CH150 Chapter 3 Ions and Ionic Compounds Chemistry
Binary ionic compounds are composed of just two elements: Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: A metal (which forms the cations) and a nonmetal (which forms the anions). In modern language, the central idea of an ionic bond is that electrons (one or more, depending on.
Covalent Bonding (Biology) — Definition & Role Expii
A metal (which forms the cations) and a nonmetal (which forms the anions). It is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a. Binary ionic compounds are composed of just two elements: Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds:.
chemistry knowledge Comparison between Covalent and Ionic Bond
Therefore, they will form ionic bond. A metal (which forms the cations) and a nonmetal (which forms the anions). Binary ionic compounds are composed of just two elements: In modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element) were transfered between the outer rings. Which pair of elements will form.
Atoms of which pair of elements will form ionic bonds in a compound
It is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a. Binary ionic compounds are composed of just two elements: Which pair of elements will form an ionic bond? Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Therefore, they will form.
Therefore, They Will Form Ionic Bond.
In modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element) were transfered between the outer rings. A metal (which forms the cations) and a nonmetal (which forms the anions). Which pair of elements will form an ionic bond? Binary ionic compounds are composed of just two elements:
Compounds Composed Of Ions Are Called Ionic Compounds (Or Salts), And Their Constituent Ions Are Held Together By Ionic Bonds:
It is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a.