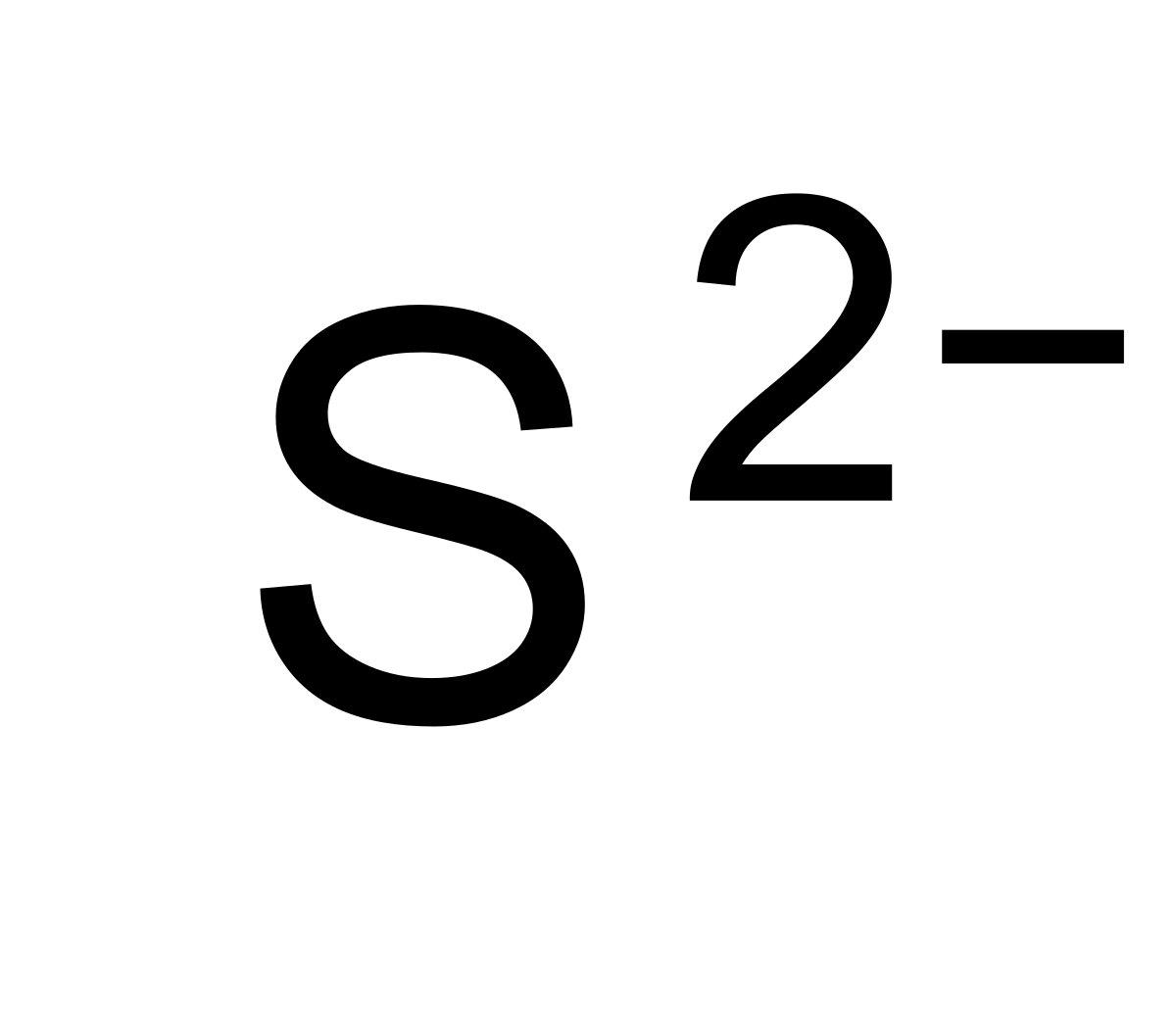What Is The Charge Of Sulfide - In your case, the sulfide anion, s2−, carries a (2 −) negative charge, which can only mean that it gained electrons. Roman numeral notation indicates charge of ion when element commonly forms more than one ion. For example, iron( ii ) has a 2+ charge;
In your case, the sulfide anion, s2−, carries a (2 −) negative charge, which can only mean that it gained electrons. For example, iron( ii ) has a 2+ charge; Roman numeral notation indicates charge of ion when element commonly forms more than one ion.
Roman numeral notation indicates charge of ion when element commonly forms more than one ion. For example, iron( ii ) has a 2+ charge; In your case, the sulfide anion, s2−, carries a (2 −) negative charge, which can only mean that it gained electrons.
Schematic illustration of the different types of interphases between
Roman numeral notation indicates charge of ion when element commonly forms more than one ion. In your case, the sulfide anion, s2−, carries a (2 −) negative charge, which can only mean that it gained electrons. For example, iron( ii ) has a 2+ charge;
Sulfide charge atilagrid
Roman numeral notation indicates charge of ion when element commonly forms more than one ion. For example, iron( ii ) has a 2+ charge; In your case, the sulfide anion, s2−, carries a (2 −) negative charge, which can only mean that it gained electrons.
Chargedischarge profiles of bismuth sulfide recorded at 2 A g −1 (a
For example, iron( ii ) has a 2+ charge; Roman numeral notation indicates charge of ion when element commonly forms more than one ion. In your case, the sulfide anion, s2−, carries a (2 −) negative charge, which can only mean that it gained electrons.
Sulfide charge jordoutdoor
In your case, the sulfide anion, s2−, carries a (2 −) negative charge, which can only mean that it gained electrons. Roman numeral notation indicates charge of ion when element commonly forms more than one ion. For example, iron( ii ) has a 2+ charge;
We can draw three inequivalent Lewis structures for carbonyl sulfide
For example, iron( ii ) has a 2+ charge; In your case, the sulfide anion, s2−, carries a (2 −) negative charge, which can only mean that it gained electrons. Roman numeral notation indicates charge of ion when element commonly forms more than one ion.
Insights into interfacial physiochemistry in sulfide solidstate
For example, iron( ii ) has a 2+ charge; In your case, the sulfide anion, s2−, carries a (2 −) negative charge, which can only mean that it gained electrons. Roman numeral notation indicates charge of ion when element commonly forms more than one ion.
SOLVEDFor each of the following ions, write the full electron
In your case, the sulfide anion, s2−, carries a (2 −) negative charge, which can only mean that it gained electrons. Roman numeral notation indicates charge of ion when element commonly forms more than one ion. For example, iron( ii ) has a 2+ charge;
Sulfate définition illustrée et explications
In your case, the sulfide anion, s2−, carries a (2 −) negative charge, which can only mean that it gained electrons. Roman numeral notation indicates charge of ion when element commonly forms more than one ion. For example, iron( ii ) has a 2+ charge;
Sulfide Wikipedia
In your case, the sulfide anion, s2−, carries a (2 −) negative charge, which can only mean that it gained electrons. For example, iron( ii ) has a 2+ charge; Roman numeral notation indicates charge of ion when element commonly forms more than one ion.
How to Write the Formula for Sulfide ion YouTube
For example, iron( ii ) has a 2+ charge; Roman numeral notation indicates charge of ion when element commonly forms more than one ion. In your case, the sulfide anion, s2−, carries a (2 −) negative charge, which can only mean that it gained electrons.
For Example, Iron( Ii ) Has A 2+ Charge;
In your case, the sulfide anion, s2−, carries a (2 −) negative charge, which can only mean that it gained electrons. Roman numeral notation indicates charge of ion when element commonly forms more than one ion.