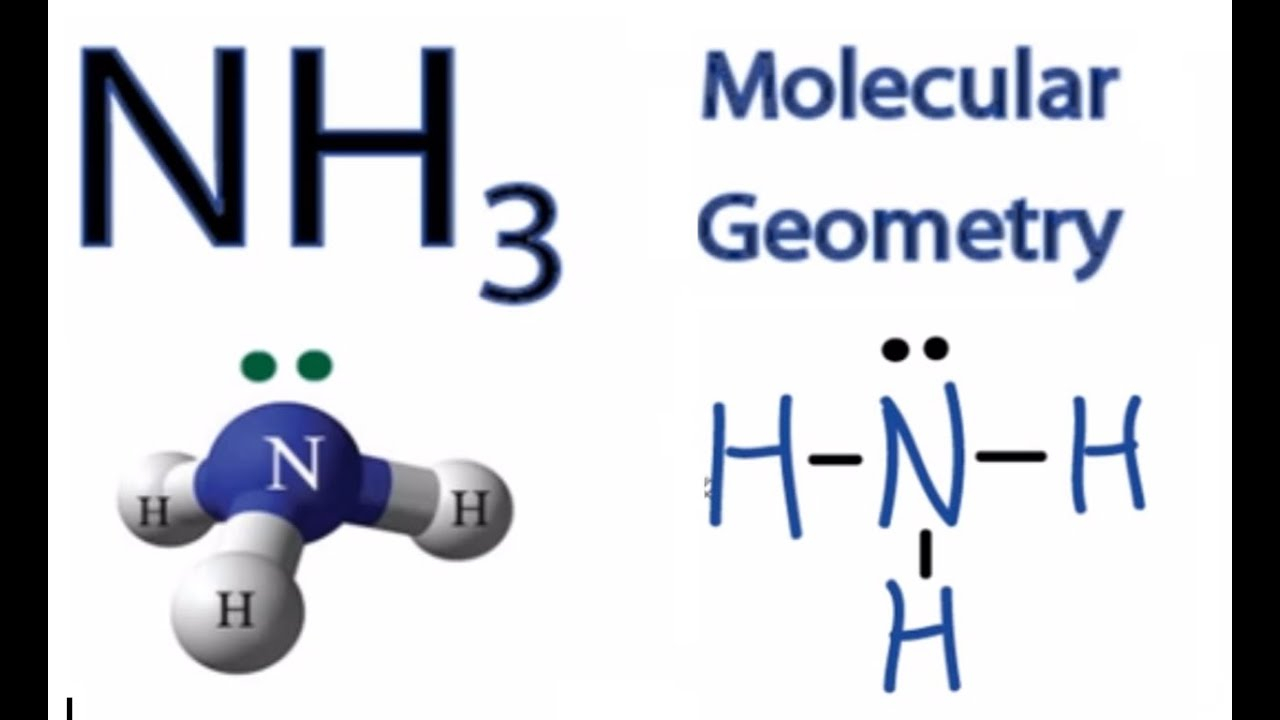
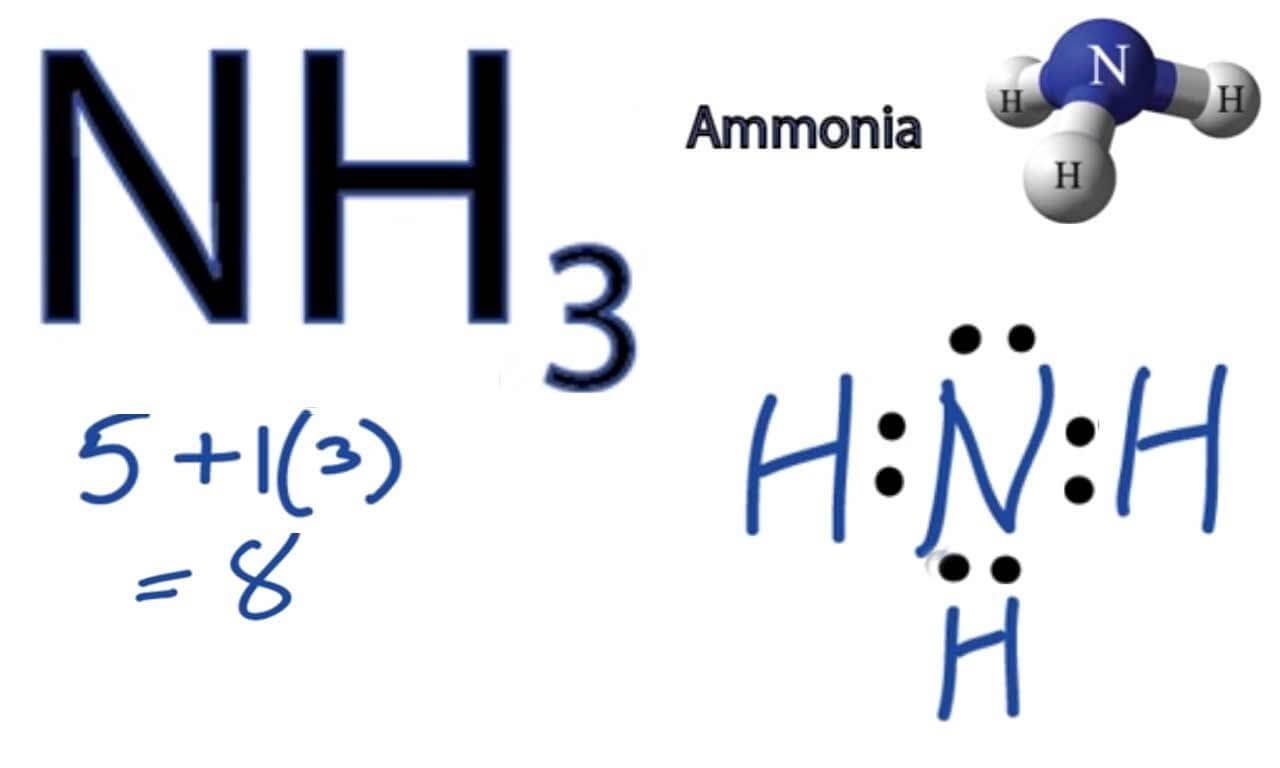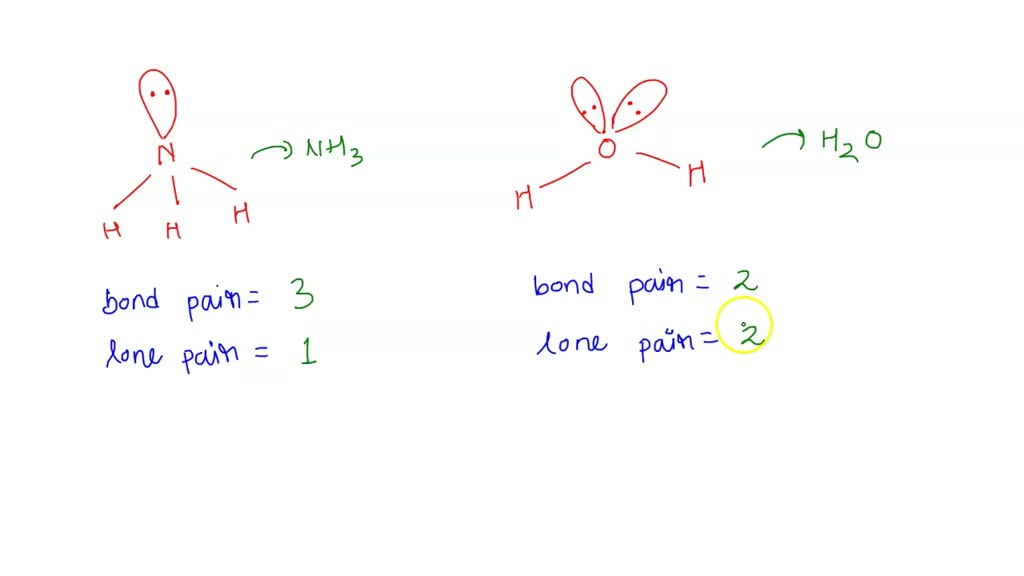What Is The Bond Angle Of Nh3 - The expected bond angle is 109.28 degrees, and the geometry is tetrahedral. In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,. Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb.
In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,. The expected bond angle is 109.28 degrees, and the geometry is tetrahedral. Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb.
The expected bond angle is 109.28 degrees, and the geometry is tetrahedral. In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,. Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb.
Lewis Diagram Of Nh3
In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,. The expected bond angle is 109.28 degrees, and the geometry is tetrahedral. Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb.
Nh3 Molecular Geometry Hybridization Bond Angle And Molecular Shape
In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,. The expected bond angle is 109.28 degrees, and the geometry is tetrahedral. Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb.
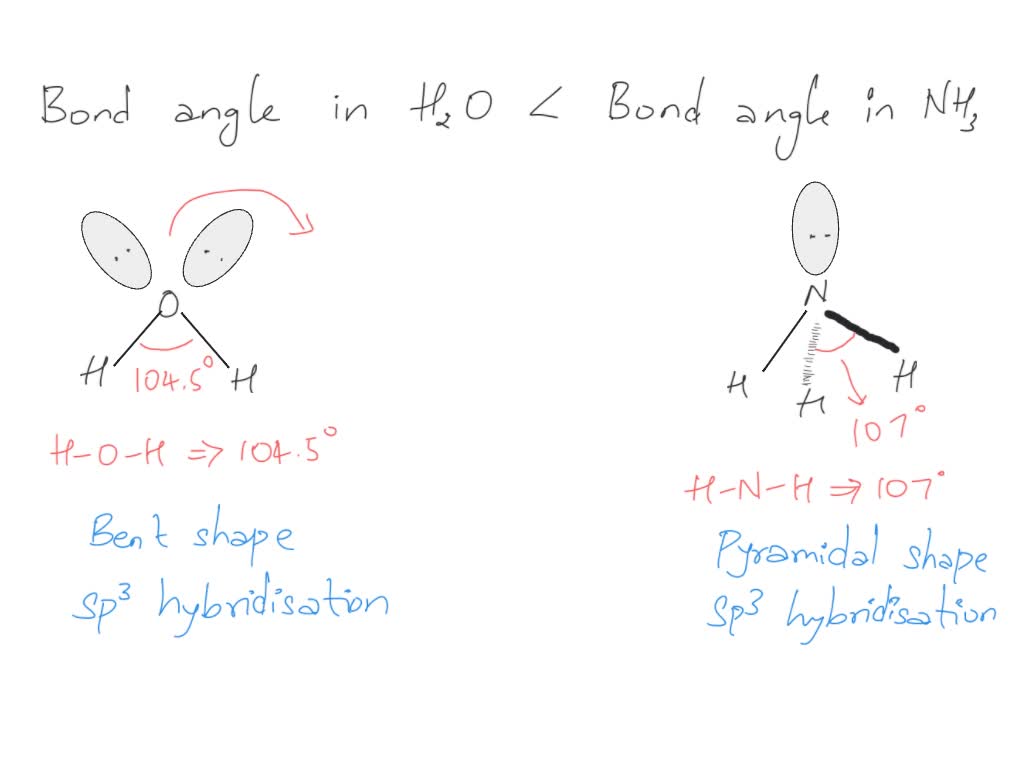
SOLVED Bond angle in Nh3 is more then in H2O . justify
The expected bond angle is 109.28 degrees, and the geometry is tetrahedral. Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,.
which is the incerrect about bond angles ? (1) NH3 > NF3 (2) NF3 > PF3
In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,. Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. The expected bond angle is 109.28 degrees, and the geometry is tetrahedral.
Solved Examine The Bond Lengths And Bond Angles For Three, 42 OFF
Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,. The expected bond angle is 109.28 degrees, and the geometry is tetrahedral.
Nh3 é Polar Ou Apolar
The expected bond angle is 109.28 degrees, and the geometry is tetrahedral. Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,.
ii) Bond angle of NH3 is than H2O. Justify
The expected bond angle is 109.28 degrees, and the geometry is tetrahedral. Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,.
Q1. arrange the following in the increasing order of bond angle nh3 nf3
In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,. Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. The expected bond angle is 109.28 degrees, and the geometry is tetrahedral.
Why does NH3 have a larger bond angle than PH3?
In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,. Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. The expected bond angle is 109.28 degrees, and the geometry is tetrahedral.
SOLVED Bond angle in nh3 is greater than bond angle in a s h 3
In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,. Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. The expected bond angle is 109.28 degrees, and the geometry is tetrahedral.
In Contrast, Ammonia Shows Trigonal Pyramidal Geometry And <109 Bond Angle,.
Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. The expected bond angle is 109.28 degrees, and the geometry is tetrahedral.