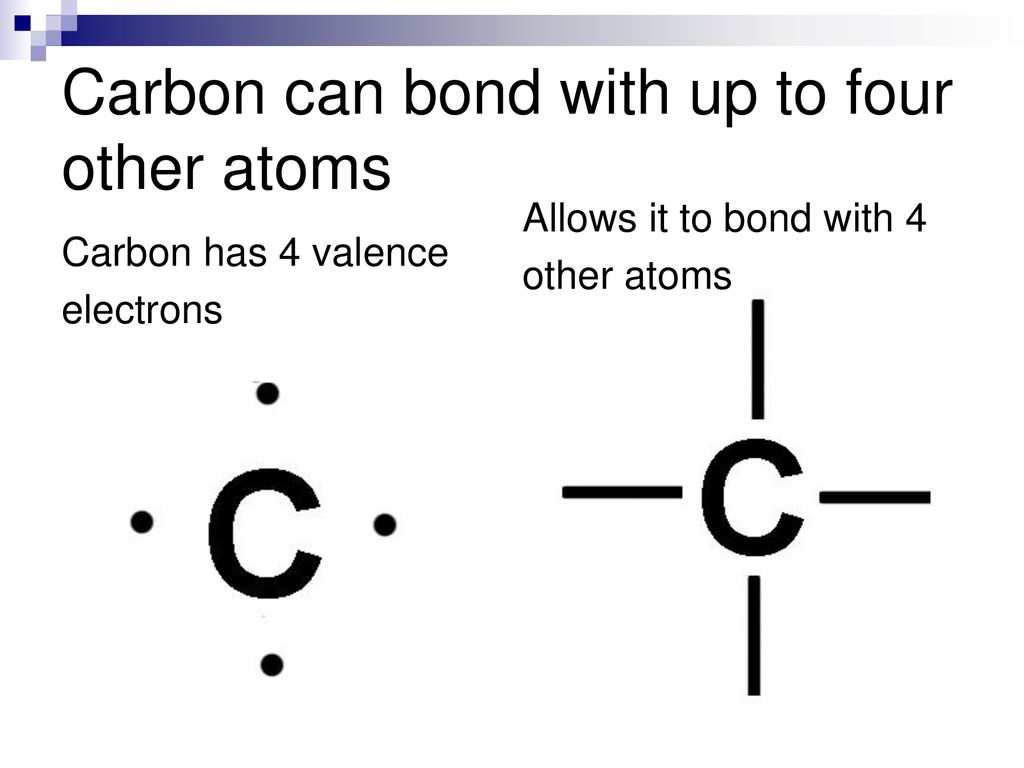
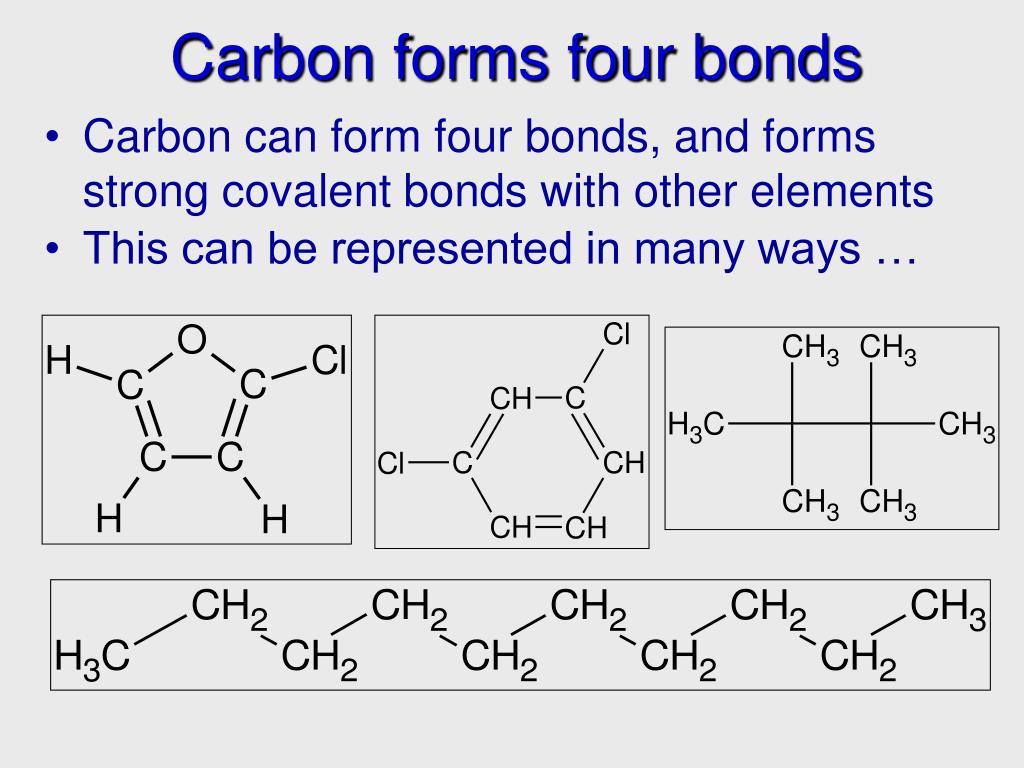
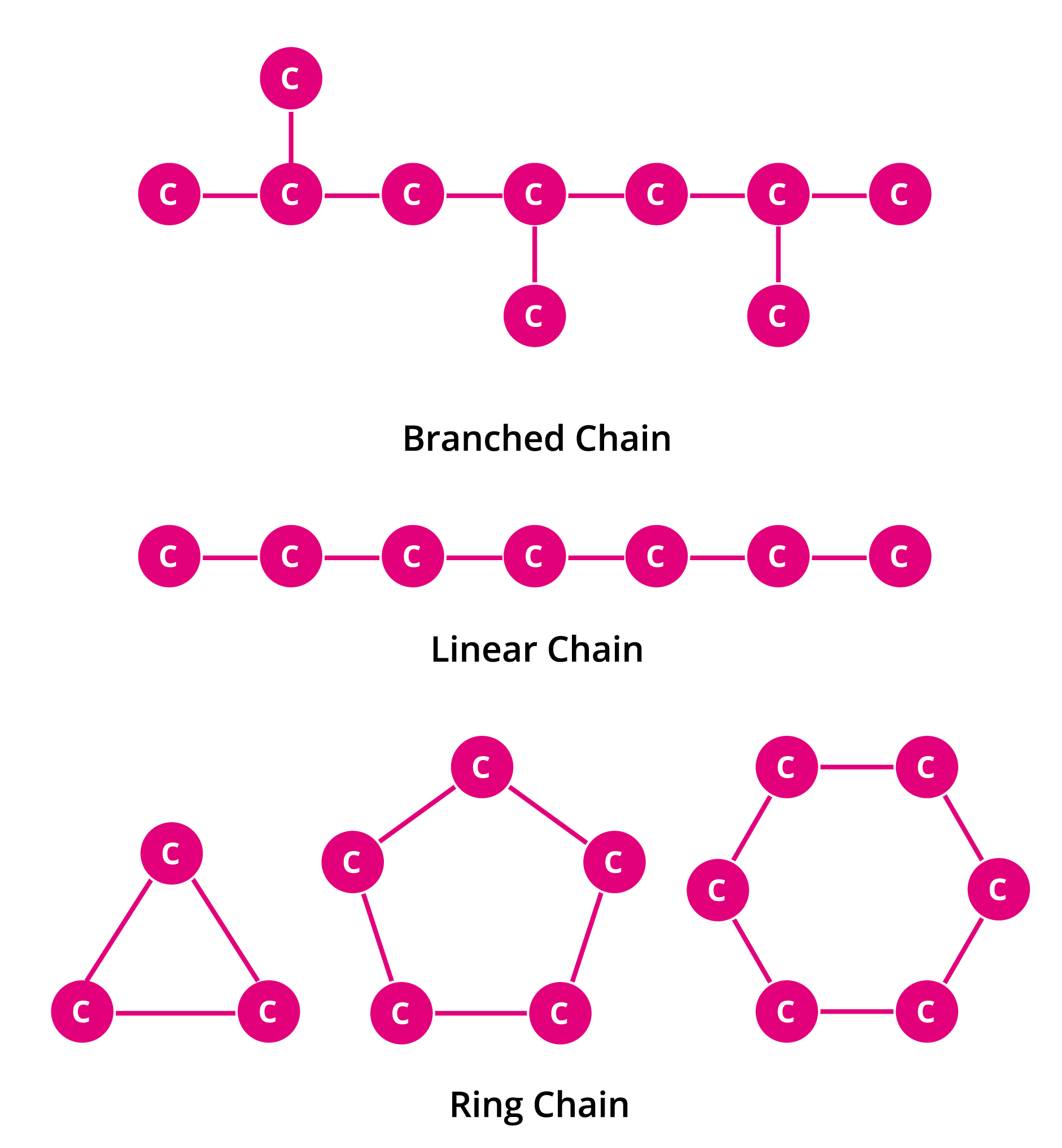
What Are The 4 Types Of Bonds Carbon Can Form - Carbon can form four single bonds, where it shares one electron. In a single bond, two carbon atoms share one pair of electrons. The simplest carbon molecule is methane (ch 4), depicted here. What are the 4 types of carbon bonds? Carbon binds to oxygen, hydrogen, and nitrogen covalently to form the many molecules important for cellular function. Carbon can form four covalent bonds to create an organic molecule. Carbon can form four types of chemical bonds: One double and two single bonds. Carbon can form single, double, or even triple bonds with other carbon atoms.
What are the 4 types of carbon bonds? One double and two single bonds. Carbon binds to oxygen, hydrogen, and nitrogen covalently to form the many molecules important for cellular function. Carbon can form four types of chemical bonds: Carbon can form single, double, or even triple bonds with other carbon atoms. The simplest carbon molecule is methane (ch 4), depicted here. Carbon can form four single bonds, where it shares one electron. Carbon can form four covalent bonds to create an organic molecule. In a single bond, two carbon atoms share one pair of electrons.
Carbon can form four covalent bonds to create an organic molecule. Carbon binds to oxygen, hydrogen, and nitrogen covalently to form the many molecules important for cellular function. The simplest carbon molecule is methane (ch 4), depicted here. Carbon can form four types of chemical bonds: In a single bond, two carbon atoms share one pair of electrons. Carbon can form four single bonds, where it shares one electron. Carbon can form single, double, or even triple bonds with other carbon atoms. One double and two single bonds. What are the 4 types of carbon bonds?
What Are The Four Types Of Bonds Carbon Can Form Design Talk
The simplest carbon molecule is methane (ch 4), depicted here. Carbon can form four covalent bonds to create an organic molecule. In a single bond, two carbon atoms share one pair of electrons. Carbon binds to oxygen, hydrogen, and nitrogen covalently to form the many molecules important for cellular function. Carbon can form single, double, or even triple bonds with.
Organic Molecules The “stuff” of life. ppt download
One double and two single bonds. Carbon binds to oxygen, hydrogen, and nitrogen covalently to form the many molecules important for cellular function. Carbon can form four covalent bonds to create an organic molecule. In a single bond, two carbon atoms share one pair of electrons. Carbon can form four single bonds, where it shares one electron.
PPT Organic Chemistry Functional Groups PowerPoint Presentation
Carbon can form single, double, or even triple bonds with other carbon atoms. Carbon binds to oxygen, hydrogen, and nitrogen covalently to form the many molecules important for cellular function. Carbon can form four covalent bonds to create an organic molecule. One double and two single bonds. The simplest carbon molecule is methane (ch 4), depicted here.
Special features of Carbon — lesson. Science State Board, Class 9.
What are the 4 types of carbon bonds? Carbon can form four covalent bonds to create an organic molecule. One double and two single bonds. Carbon can form four types of chemical bonds: Carbon can form single, double, or even triple bonds with other carbon atoms.
The 4 Types of Bonds Carbon Can Form Video & Lesson Transcript
One double and two single bonds. Carbon can form single, double, or even triple bonds with other carbon atoms. The simplest carbon molecule is methane (ch 4), depicted here. In a single bond, two carbon atoms share one pair of electrons. Carbon can form four covalent bonds to create an organic molecule.
[Class 10 Chemistry] Bonding in Carbon Atoms Covalent Bonds
In a single bond, two carbon atoms share one pair of electrons. Carbon can form four types of chemical bonds: The simplest carbon molecule is methane (ch 4), depicted here. Carbon can form single, double, or even triple bonds with other carbon atoms. Carbon binds to oxygen, hydrogen, and nitrogen covalently to form the many molecules important for cellular function.
Double Covalent Bond Covalent Bonding Quiz ProProfs Quiz, The
The simplest carbon molecule is methane (ch 4), depicted here. Carbon can form four types of chemical bonds: Carbon can form four single bonds, where it shares one electron. One double and two single bonds. What are the 4 types of carbon bonds?
Why carbon does not form four bonds with another carbon?/chemical
One double and two single bonds. In a single bond, two carbon atoms share one pair of electrons. Carbon binds to oxygen, hydrogen, and nitrogen covalently to form the many molecules important for cellular function. Carbon can form four covalent bonds to create an organic molecule. Carbon can form single, double, or even triple bonds with other carbon atoms.
Carbon Compounds and Examples
The simplest carbon molecule is methane (ch 4), depicted here. Carbon can form four covalent bonds to create an organic molecule. Carbon can form four types of chemical bonds: In a single bond, two carbon atoms share one pair of electrons. One double and two single bonds.
[Class 10 Chemistry] Bonding in Carbon Atoms Covalent Bonds
The simplest carbon molecule is methane (ch 4), depicted here. In a single bond, two carbon atoms share one pair of electrons. Carbon can form four types of chemical bonds: One double and two single bonds. Carbon can form single, double, or even triple bonds with other carbon atoms.
In A Single Bond, Two Carbon Atoms Share One Pair Of Electrons.
Carbon can form four single bonds, where it shares one electron. Carbon binds to oxygen, hydrogen, and nitrogen covalently to form the many molecules important for cellular function. Carbon can form four types of chemical bonds: One double and two single bonds.
Carbon Can Form Single, Double, Or Even Triple Bonds With Other Carbon Atoms.
The simplest carbon molecule is methane (ch 4), depicted here. What are the 4 types of carbon bonds? Carbon can form four covalent bonds to create an organic molecule.


![[Class 10 Chemistry] Bonding in Carbon Atoms Covalent Bonds](https://d1avenlh0i1xmr.cloudfront.net/medium/65382287-b5ac-4ea6-8a57-1f5a107b172c/a-carbon-atom---teachoo.jpg)



![[Class 10 Chemistry] Bonding in Carbon Atoms Covalent Bonds](https://d1avenlh0i1xmr.cloudfront.net/large/6f2704db-da18-4198-8dd5-4dc81ae3ac22/carbon-sharing-electrons-with-hydrogen---teachoo.jpg)