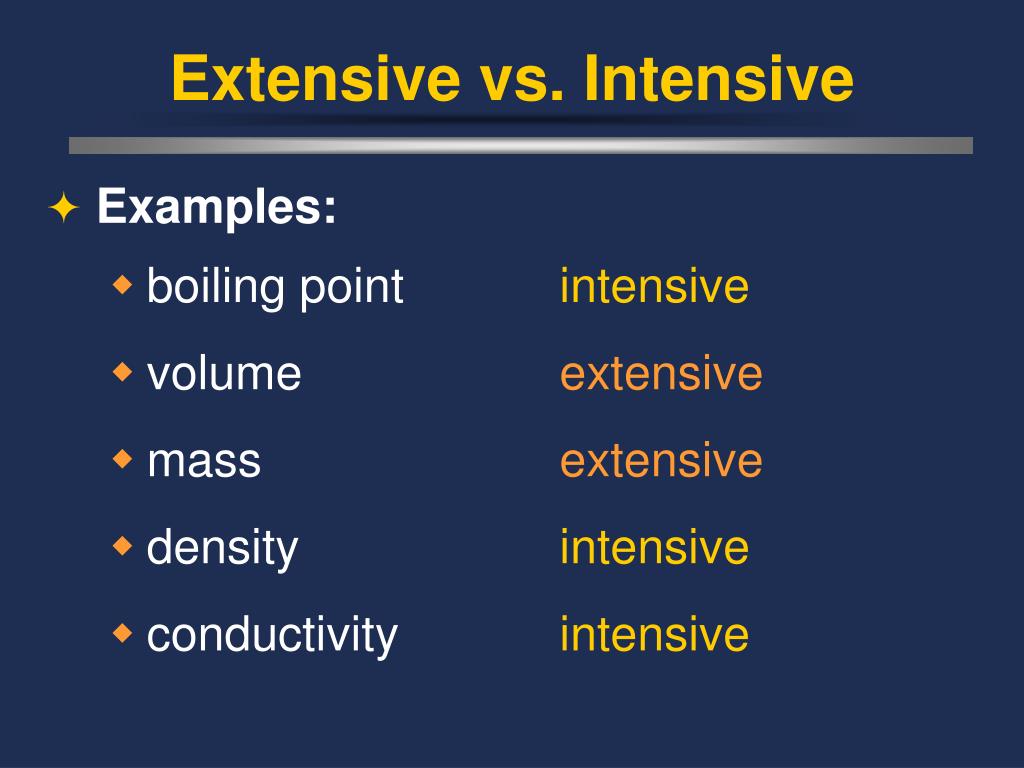
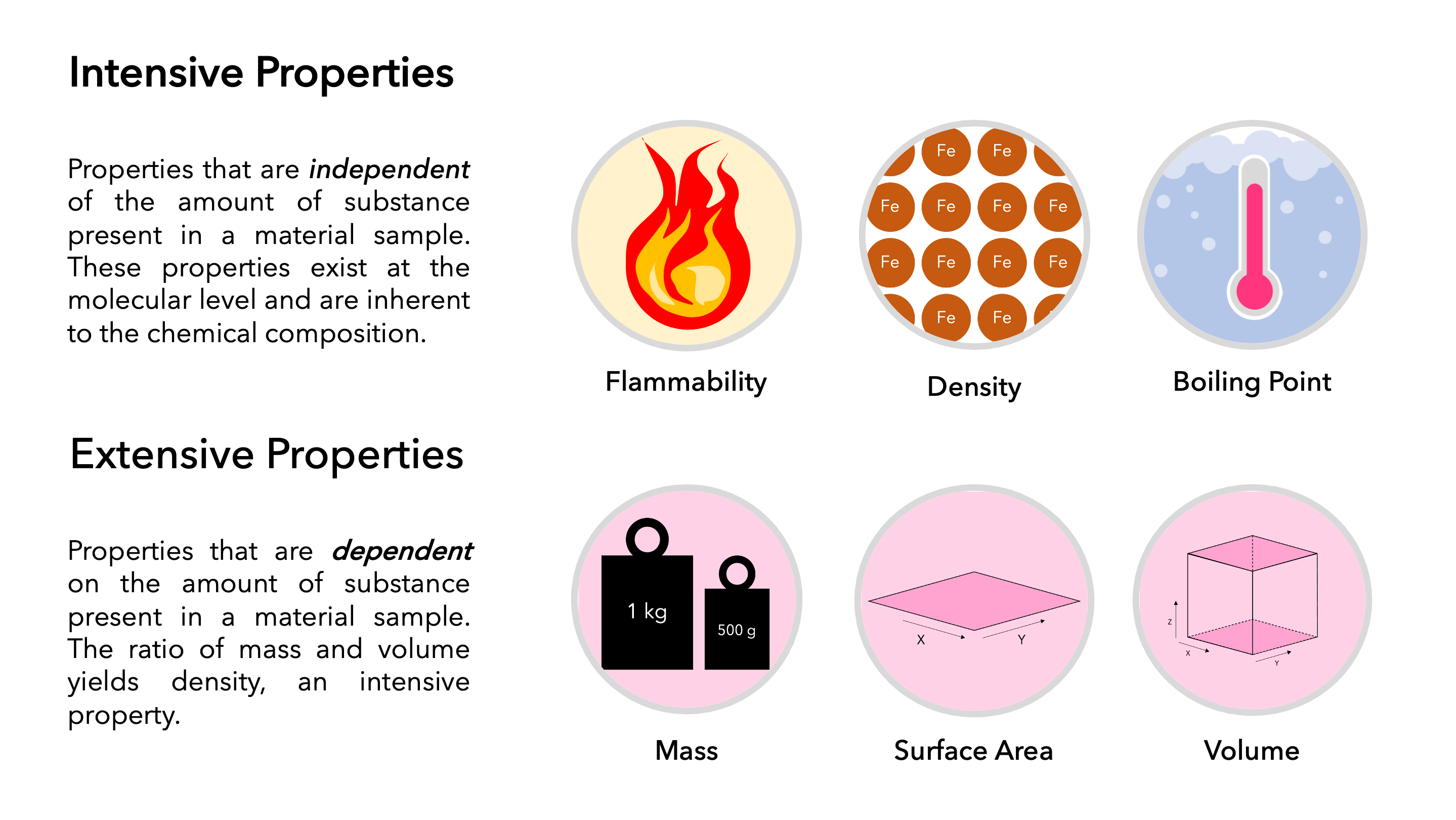
Extensive Vs Intensive Properties - Extensive and intensive properties are the two types of physical properties of matter. An intensive property is a property of matter that. An extensive property is a property that depends on the amount of matter in a sample. Extensive properties vary with the amount of the substance and include mass, weight, and volume. The two types of physical properties of matter are intensive properties and extensive properties. The ratio of two extensive properties of the same object or system is an intensive property. Intensive properties do not depend on the quantity of matter. For example, the ratio of an object's mass and volume, which are two extensive properties, is. Intensive properties do not depend on the amount of matter in a substance. Mass and volume are examples of extensive properties.
Extensive properties vary with the amount of the substance and include mass, weight, and volume. Intensive properties, in contrast, do not depend on the amount of the substance; Intensive properties do not depend on the quantity of matter. Mass and volume are examples of extensive properties. An intensive property is a property of matter that. The ratio of two extensive properties of the same object or system is an intensive property. For example, the ratio of an object's mass and volume, which are two extensive properties, is. An extensive property is a property that depends on the amount of matter in a sample. The two types of physical properties of matter are intensive properties and extensive properties. Intensive properties do not depend on the amount of matter in a substance.
Extensive properties vary with the amount of the substance and include mass, weight, and volume. For example, the ratio of an object's mass and volume, which are two extensive properties, is. Intensive properties do not depend on the amount of matter in a substance. Intensive properties, in contrast, do not depend on the amount of the substance; An extensive property is a property that depends on the amount of matter in a sample. Intensive properties do not depend on the quantity of matter. An intensive property is a property of matter that. Extensive and intensive properties are the two types of physical properties of matter. The two types of physical properties of matter are intensive properties and extensive properties. Mass and volume are examples of extensive properties.
The Difference Between Intensive and Extensive Properties
Extensive and intensive properties are the two types of physical properties of matter. Extensive properties vary with the amount of the substance and include mass, weight, and volume. The two types of physical properties of matter are intensive properties and extensive properties. The ratio of two extensive properties of the same object or system is an intensive property. Intensive properties.
Difference Between Intensive Property and Extensive Property
Extensive properties vary with the amount of the substance and include mass, weight, and volume. Mass and volume are examples of extensive properties. Intensive properties do not depend on the quantity of matter. Intensive properties do not depend on the amount of matter in a substance. Extensive and intensive properties are the two types of physical properties of matter.
Extensive Properties Vs Intensive Properties
For example, the ratio of an object's mass and volume, which are two extensive properties, is. The two types of physical properties of matter are intensive properties and extensive properties. Intensive properties do not depend on the amount of matter in a substance. Intensive properties do not depend on the quantity of matter. Mass and volume are examples of extensive.
PPT Properties & Changes in Matter Extensive vs. Intensive Physical
The two types of physical properties of matter are intensive properties and extensive properties. Intensive properties, in contrast, do not depend on the amount of the substance; For example, the ratio of an object's mass and volume, which are two extensive properties, is. Intensive properties do not depend on the quantity of matter. Extensive properties vary with the amount of.
Intensive vs. Extensive Property What's the Difference? • 7ESL
Extensive properties vary with the amount of the substance and include mass, weight, and volume. Extensive and intensive properties are the two types of physical properties of matter. The two types of physical properties of matter are intensive properties and extensive properties. Intensive properties do not depend on the amount of matter in a substance. An intensive property is a.
Intensive and extensive properties YouTube
Extensive properties vary with the amount of the substance and include mass, weight, and volume. The two types of physical properties of matter are intensive properties and extensive properties. The ratio of two extensive properties of the same object or system is an intensive property. Intensive properties do not depend on the amount of matter in a substance. Intensive properties.
Extensive vs. Intensive Properties — Overview & Examples Expii
Intensive properties do not depend on the amount of matter in a substance. Intensive properties do not depend on the quantity of matter. The ratio of two extensive properties of the same object or system is an intensive property. Mass and volume are examples of extensive properties. Extensive properties vary with the amount of the substance and include mass, weight,.
Difference Between Intensive and Extensive Properties Definition
Extensive and intensive properties are the two types of physical properties of matter. An intensive property is a property of matter that. Intensive properties, in contrast, do not depend on the amount of the substance; Mass and volume are examples of extensive properties. An extensive property is a property that depends on the amount of matter in a sample.
Intensive vs. Extensive Property What's the Difference? • 7ESL
For example, the ratio of an object's mass and volume, which are two extensive properties, is. Mass and volume are examples of extensive properties. Extensive and intensive properties are the two types of physical properties of matter. Intensive properties, in contrast, do not depend on the amount of the substance; Intensive properties do not depend on the quantity of matter.
Extensive and Intensive Properties Study Guide Inspirit
Extensive properties vary with the amount of the substance and include mass, weight, and volume. Intensive properties do not depend on the amount of matter in a substance. The two types of physical properties of matter are intensive properties and extensive properties. The ratio of two extensive properties of the same object or system is an intensive property. For example,.
The Ratio Of Two Extensive Properties Of The Same Object Or System Is An Intensive Property.
An intensive property is a property of matter that. Intensive properties do not depend on the amount of matter in a substance. Intensive properties, in contrast, do not depend on the amount of the substance; Extensive properties vary with the amount of the substance and include mass, weight, and volume.
Intensive Properties Do Not Depend On The Quantity Of Matter.
Extensive and intensive properties are the two types of physical properties of matter. For example, the ratio of an object's mass and volume, which are two extensive properties, is. An extensive property is a property that depends on the amount of matter in a sample. Mass and volume are examples of extensive properties.
/intensive-vs-extensive-properties-604133-v3-5b55fb394cedfd0037117796.png)