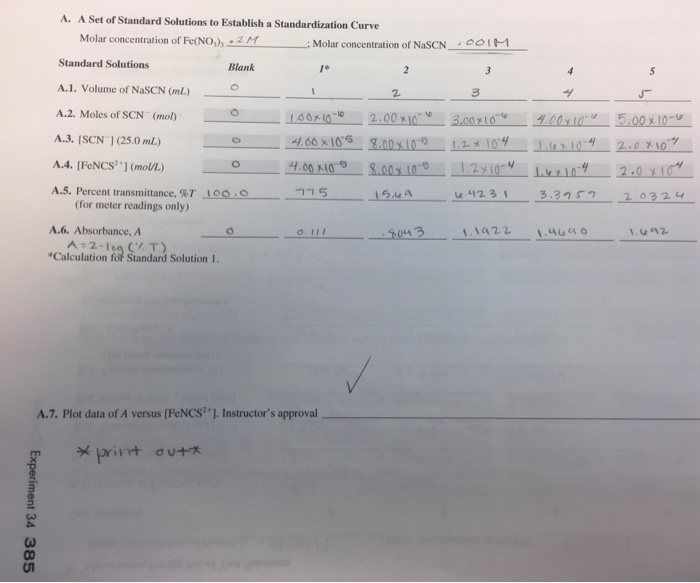
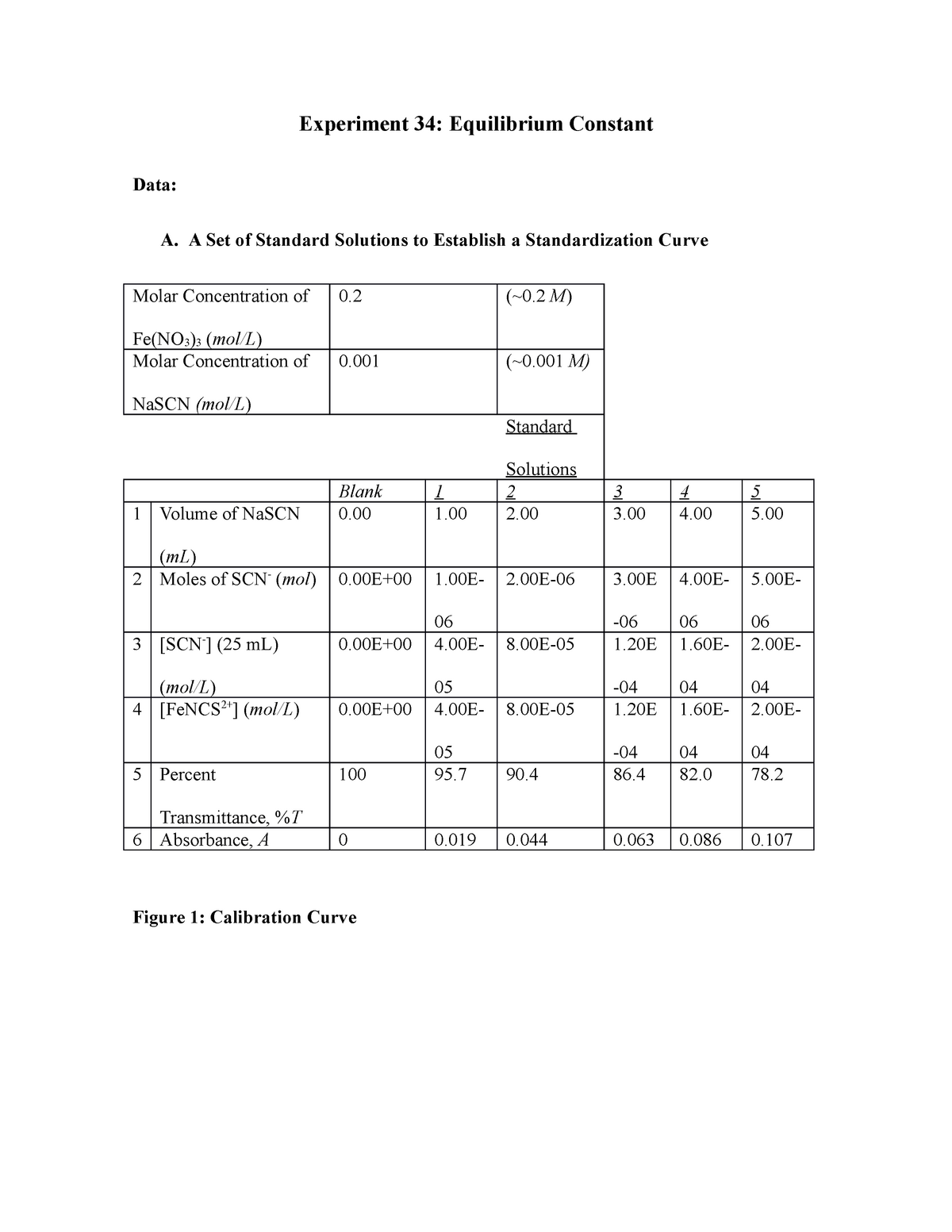
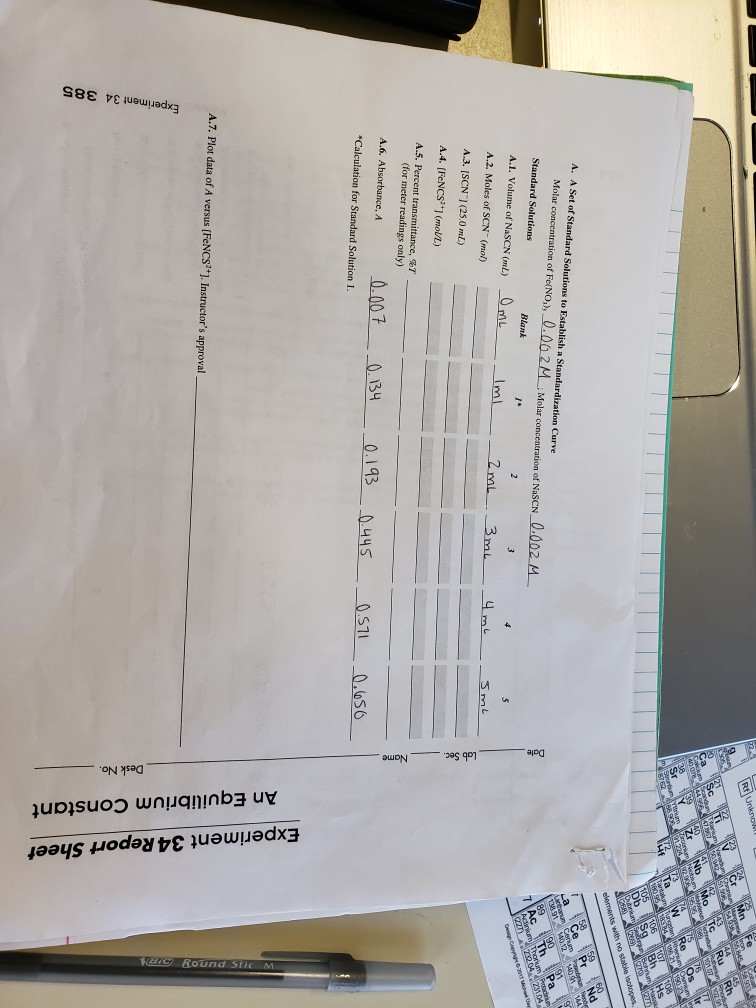
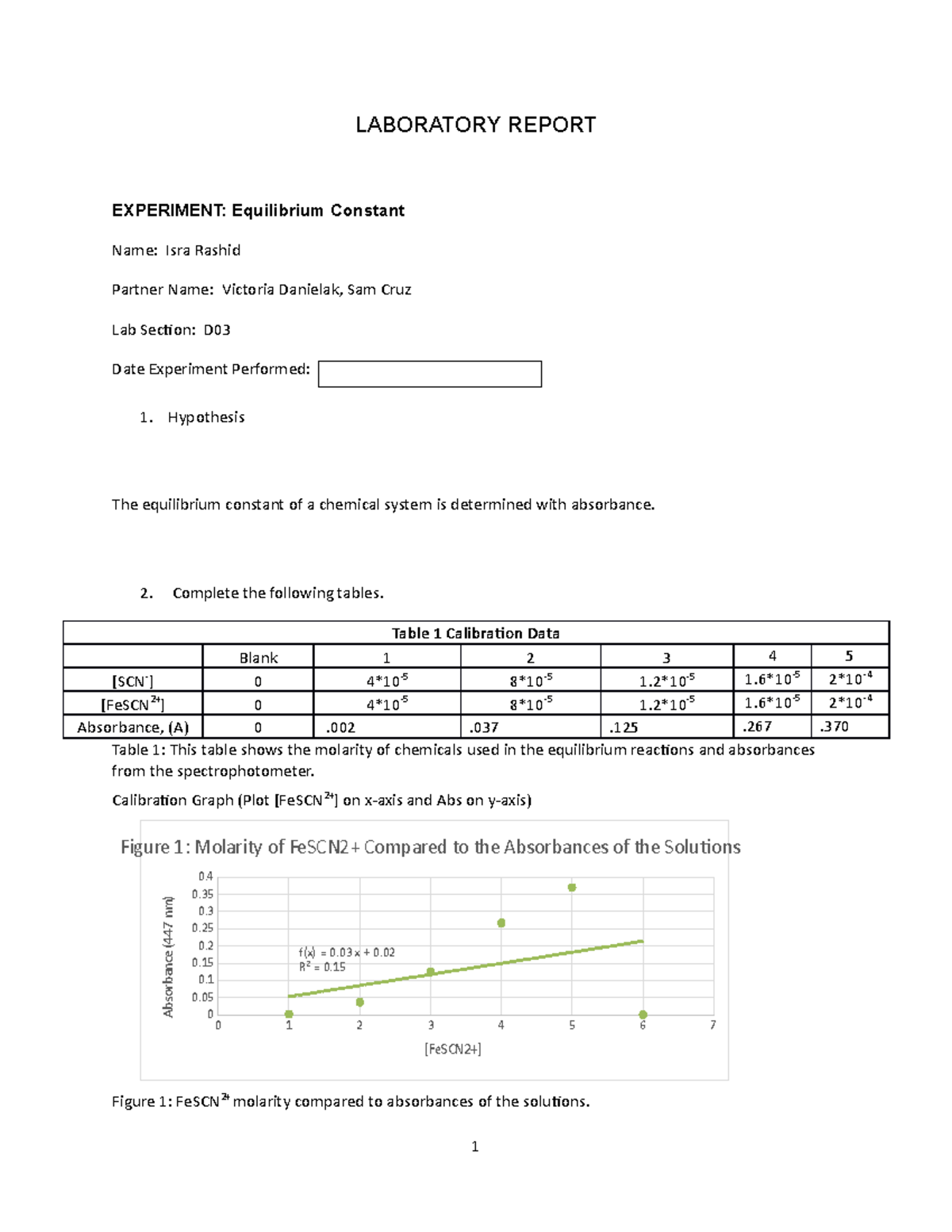
Experiment 34 An Equilibrium Constant Report Sheet - Equilibrium favors the products, while a small k value indicates that the equilibrium favors the reactants. Experiment 34, detailed in this report,. Hypothesis/objective to use a spectrophotometer to determine the equilibrium constant of a chemical system, to use graphing. What is an equilibrium constant? A numerical value representing the ratio of products to reactants at equilibrium.
Equilibrium favors the products, while a small k value indicates that the equilibrium favors the reactants. Experiment 34, detailed in this report,. A numerical value representing the ratio of products to reactants at equilibrium. What is an equilibrium constant? Hypothesis/objective to use a spectrophotometer to determine the equilibrium constant of a chemical system, to use graphing.
What is an equilibrium constant? Equilibrium favors the products, while a small k value indicates that the equilibrium favors the reactants. Hypothesis/objective to use a spectrophotometer to determine the equilibrium constant of a chemical system, to use graphing. Experiment 34, detailed in this report,. A numerical value representing the ratio of products to reactants at equilibrium.
Experiment 34 An Equilibrium Constant YouTube
Equilibrium favors the products, while a small k value indicates that the equilibrium favors the reactants. What is an equilibrium constant? Experiment 34, detailed in this report,. A numerical value representing the ratio of products to reactants at equilibrium. Hypothesis/objective to use a spectrophotometer to determine the equilibrium constant of a chemical system, to use graphing.
Solved Please help me with part C. I posted part A, B and
Hypothesis/objective to use a spectrophotometer to determine the equilibrium constant of a chemical system, to use graphing. Experiment 34, detailed in this report,. What is an equilibrium constant? A numerical value representing the ratio of products to reactants at equilibrium. Equilibrium favors the products, while a small k value indicates that the equilibrium favors the reactants.
Solved The next questions are related to Experiment 34.
Experiment 34, detailed in this report,. Equilibrium favors the products, while a small k value indicates that the equilibrium favors the reactants. A numerical value representing the ratio of products to reactants at equilibrium. Hypothesis/objective to use a spectrophotometer to determine the equilibrium constant of a chemical system, to use graphing. What is an equilibrium constant?
Solved Experiment 34 ratory Assignment An Equilibrium
Experiment 34, detailed in this report,. What is an equilibrium constant? A numerical value representing the ratio of products to reactants at equilibrium. Equilibrium favors the products, while a small k value indicates that the equilibrium favors the reactants. Hypothesis/objective to use a spectrophotometer to determine the equilibrium constant of a chemical system, to use graphing.
Experiment 34 An Equilibrium Constant
What is an equilibrium constant? Hypothesis/objective to use a spectrophotometer to determine the equilibrium constant of a chemical system, to use graphing. Experiment 34, detailed in this report,. A numerical value representing the ratio of products to reactants at equilibrium. Equilibrium favors the products, while a small k value indicates that the equilibrium favors the reactants.
Solved Experiment 34 Prelaboratory Assignment An Equilibrium
What is an equilibrium constant? A numerical value representing the ratio of products to reactants at equilibrium. Hypothesis/objective to use a spectrophotometer to determine the equilibrium constant of a chemical system, to use graphing. Experiment 34, detailed in this report,. Equilibrium favors the products, while a small k value indicates that the equilibrium favors the reactants.
Equilibrium Constant Lab Answers
Hypothesis/objective to use a spectrophotometer to determine the equilibrium constant of a chemical system, to use graphing. Equilibrium favors the products, while a small k value indicates that the equilibrium favors the reactants. What is an equilibrium constant? Experiment 34, detailed in this report,. A numerical value representing the ratio of products to reactants at equilibrium.
LAB Report; Labster Equilibrium GENERAL CHEMISTRY 2 EXPERIMENT NO. 5
Experiment 34, detailed in this report,. Hypothesis/objective to use a spectrophotometer to determine the equilibrium constant of a chemical system, to use graphing. A numerical value representing the ratio of products to reactants at equilibrium. What is an equilibrium constant? Equilibrium favors the products, while a small k value indicates that the equilibrium favors the reactants.
Experiment 34 Report Sheet An Equilibrium Constant
What is an equilibrium constant? Experiment 34, detailed in this report,. A numerical value representing the ratio of products to reactants at equilibrium. Hypothesis/objective to use a spectrophotometer to determine the equilibrium constant of a chemical system, to use graphing. Equilibrium favors the products, while a small k value indicates that the equilibrium favors the reactants.
Exp 34 Equilibrium Constant numbers only LABORATORY REPORT EXPERIMENT
Equilibrium favors the products, while a small k value indicates that the equilibrium favors the reactants. A numerical value representing the ratio of products to reactants at equilibrium. Experiment 34, detailed in this report,. Hypothesis/objective to use a spectrophotometer to determine the equilibrium constant of a chemical system, to use graphing. What is an equilibrium constant?
What Is An Equilibrium Constant?
Equilibrium favors the products, while a small k value indicates that the equilibrium favors the reactants. A numerical value representing the ratio of products to reactants at equilibrium. Hypothesis/objective to use a spectrophotometer to determine the equilibrium constant of a chemical system, to use graphing. Experiment 34, detailed in this report,.