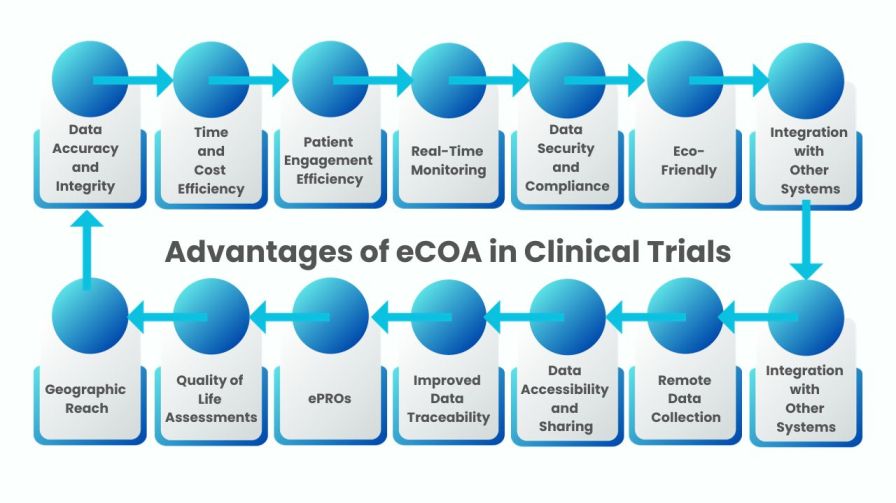
Ecoa Full Form In Clinical Trials - Ecoa stands for electronic capture of clinical outcome assessments, coas. Coas are measures of how a patient feels, functions or. Ecoa (electronic clinical outcome assessment) is a digital version of a coa (clinical outcome assessment), which measures and records how a.
Ecoa (electronic clinical outcome assessment) is a digital version of a coa (clinical outcome assessment), which measures and records how a. Ecoa stands for electronic capture of clinical outcome assessments, coas. Coas are measures of how a patient feels, functions or.
Ecoa (electronic clinical outcome assessment) is a digital version of a coa (clinical outcome assessment), which measures and records how a. Coas are measures of how a patient feels, functions or. Ecoa stands for electronic capture of clinical outcome assessments, coas.
ERT Announces eCOA Multimedia, Enhancing Patient Data Capture with
Ecoa (electronic clinical outcome assessment) is a digital version of a coa (clinical outcome assessment), which measures and records how a. Coas are measures of how a patient feels, functions or. Ecoa stands for electronic capture of clinical outcome assessments, coas.
eCOA in Clinical Trials A GameChanger for Data Collection
Ecoa stands for electronic capture of clinical outcome assessments, coas. Ecoa (electronic clinical outcome assessment) is a digital version of a coa (clinical outcome assessment), which measures and records how a. Coas are measures of how a patient feels, functions or.
eCOA Clinical Trials Solution Merative
Ecoa stands for electronic capture of clinical outcome assessments, coas. Coas are measures of how a patient feels, functions or. Ecoa (electronic clinical outcome assessment) is a digital version of a coa (clinical outcome assessment), which measures and records how a.
How To Apply For A Clinical Trial Miami Clinical Research
Coas are measures of how a patient feels, functions or. Ecoa (electronic clinical outcome assessment) is a digital version of a coa (clinical outcome assessment), which measures and records how a. Ecoa stands for electronic capture of clinical outcome assessments, coas.
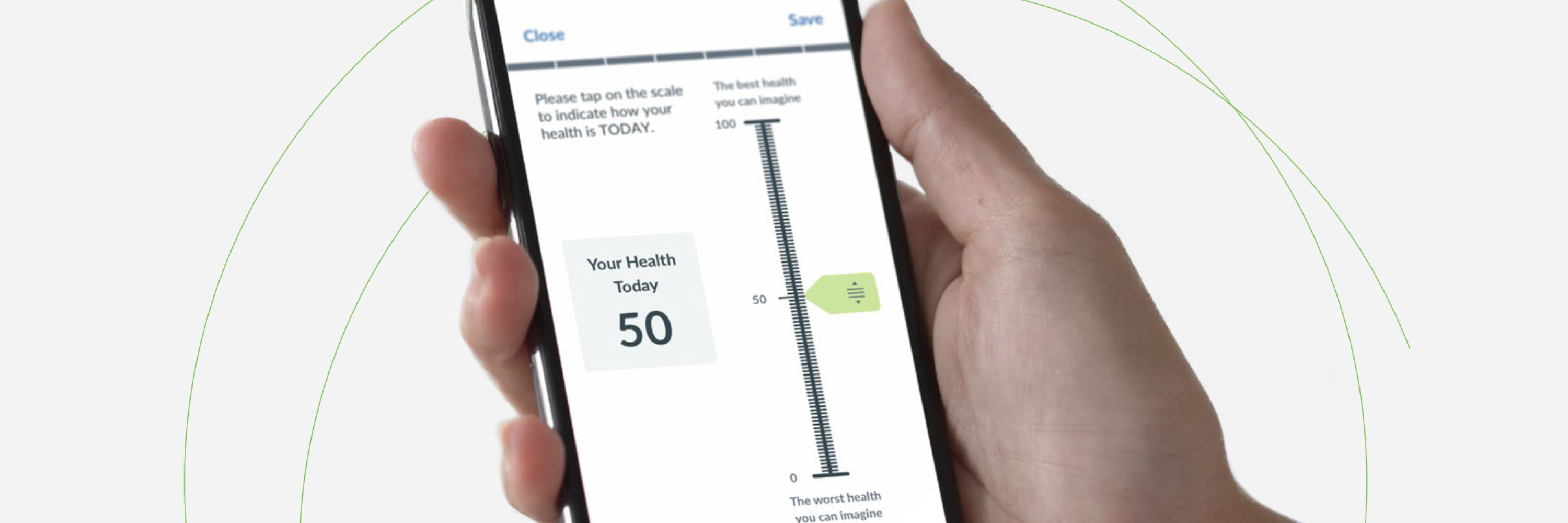
What is an eCOA and ePRO?
Ecoa (electronic clinical outcome assessment) is a digital version of a coa (clinical outcome assessment), which measures and records how a. Ecoa stands for electronic capture of clinical outcome assessments, coas. Coas are measures of how a patient feels, functions or.
What is eCOA in clinical trials? Suvoda
Coas are measures of how a patient feels, functions or. Ecoa stands for electronic capture of clinical outcome assessments, coas. Ecoa (electronic clinical outcome assessment) is a digital version of a coa (clinical outcome assessment), which measures and records how a.
Accelerating eCOA Implementation Benefits Decentralized Clinical Trials
Ecoa stands for electronic capture of clinical outcome assessments, coas. Ecoa (electronic clinical outcome assessment) is a digital version of a coa (clinical outcome assessment), which measures and records how a. Coas are measures of how a patient feels, functions or.
eCOA Clinical Trials Solution Merative
Ecoa (electronic clinical outcome assessment) is a digital version of a coa (clinical outcome assessment), which measures and records how a. Coas are measures of how a patient feels, functions or. Ecoa stands for electronic capture of clinical outcome assessments, coas.
Accelerating eCOA Implementation Benefits Decentralized Clinical Trials
Ecoa (electronic clinical outcome assessment) is a digital version of a coa (clinical outcome assessment), which measures and records how a. Ecoa stands for electronic capture of clinical outcome assessments, coas. Coas are measures of how a patient feels, functions or.
Gather eCOA Clinical Trial Platform Exco InTouch nuom
Ecoa (electronic clinical outcome assessment) is a digital version of a coa (clinical outcome assessment), which measures and records how a. Coas are measures of how a patient feels, functions or. Ecoa stands for electronic capture of clinical outcome assessments, coas.
Ecoa Stands For Electronic Capture Of Clinical Outcome Assessments, Coas.
Coas are measures of how a patient feels, functions or. Ecoa (electronic clinical outcome assessment) is a digital version of a coa (clinical outcome assessment), which measures and records how a.






.jpg)
