Barium Chloride Sodium Sulfate - Let's balance this equation using the inspection method. Sodium sulphate chemically reacts with barium chloride in the form of their aqueous solution to form a white precipitate of barium. Give the equation for the. For each element, we check if the. When barium chloride is made to react with sodium sulphate, barium sulphate and sodium chloride are formed. When solutions of barium chloride (aq) and sodium sulfate (aq) are mixed, what is/are the spectator ion (s)?
Sodium sulphate chemically reacts with barium chloride in the form of their aqueous solution to form a white precipitate of barium. When solutions of barium chloride (aq) and sodium sulfate (aq) are mixed, what is/are the spectator ion (s)? When barium chloride is made to react with sodium sulphate, barium sulphate and sodium chloride are formed. Let's balance this equation using the inspection method. For each element, we check if the. Give the equation for the.
Give the equation for the. Sodium sulphate chemically reacts with barium chloride in the form of their aqueous solution to form a white precipitate of barium. Let's balance this equation using the inspection method. When barium chloride is made to react with sodium sulphate, barium sulphate and sodium chloride are formed. For each element, we check if the. When solutions of barium chloride (aq) and sodium sulfate (aq) are mixed, what is/are the spectator ion (s)?
Reaction Between Sodium Sulphate and Barium Chloride Solution MeitY
Sodium sulphate chemically reacts with barium chloride in the form of their aqueous solution to form a white precipitate of barium. Give the equation for the. Let's balance this equation using the inspection method. When barium chloride is made to react with sodium sulphate, barium sulphate and sodium chloride are formed. When solutions of barium chloride (aq) and sodium sulfate.
science chemistry precipitation reaction barium sulfate Fundamental
When solutions of barium chloride (aq) and sodium sulfate (aq) are mixed, what is/are the spectator ion (s)? Sodium sulphate chemically reacts with barium chloride in the form of their aqueous solution to form a white precipitate of barium. When barium chloride is made to react with sodium sulphate, barium sulphate and sodium chloride are formed. For each element, we.
Question Video Identifying the Result of Adding Barium Chloride
When solutions of barium chloride (aq) and sodium sulfate (aq) are mixed, what is/are the spectator ion (s)? When barium chloride is made to react with sodium sulphate, barium sulphate and sodium chloride are formed. Let's balance this equation using the inspection method. For each element, we check if the. Sodium sulphate chemically reacts with barium chloride in the form.
barium chloride + sodium sulfate YouTube
Give the equation for the. When barium chloride is made to react with sodium sulphate, barium sulphate and sodium chloride are formed. When solutions of barium chloride (aq) and sodium sulfate (aq) are mixed, what is/are the spectator ion (s)? For each element, we check if the. Sodium sulphate chemically reacts with barium chloride in the form of their aqueous.
Combining Barium Chloride and Sodium Sulphate Stock Image C036/3661
When barium chloride is made to react with sodium sulphate, barium sulphate and sodium chloride are formed. When solutions of barium chloride (aq) and sodium sulfate (aq) are mixed, what is/are the spectator ion (s)? Give the equation for the. For each element, we check if the. Let's balance this equation using the inspection method.
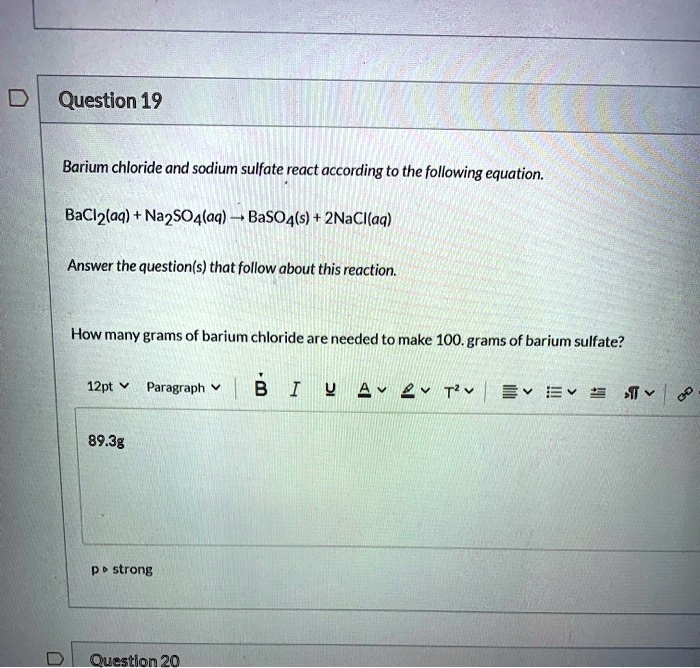
SOLVED Question 19 Barium chloride and sodium sulfate react according
When barium chloride is made to react with sodium sulphate, barium sulphate and sodium chloride are formed. For each element, we check if the. When solutions of barium chloride (aq) and sodium sulfate (aq) are mixed, what is/are the spectator ion (s)? Sodium sulphate chemically reacts with barium chloride in the form of their aqueous solution to form a white.
science chemistry precipitation reaction barium sulfate Fundamental
Sodium sulphate chemically reacts with barium chloride in the form of their aqueous solution to form a white precipitate of barium. Let's balance this equation using the inspection method. When solutions of barium chloride (aq) and sodium sulfate (aq) are mixed, what is/are the spectator ion (s)? When barium chloride is made to react with sodium sulphate, barium sulphate and.
Net Ionic Equation Of Sodium Sulfate And Barium Hydroxide 35+ Pages
Sodium sulphate chemically reacts with barium chloride in the form of their aqueous solution to form a white precipitate of barium. When solutions of barium chloride (aq) and sodium sulfate (aq) are mixed, what is/are the spectator ion (s)? For each element, we check if the. When barium chloride is made to react with sodium sulphate, barium sulphate and sodium.
Combining Barium Chloride and Sodium Sulphate Stock Image C036/3660
Let's balance this equation using the inspection method. Sodium sulphate chemically reacts with barium chloride in the form of their aqueous solution to form a white precipitate of barium. When barium chloride is made to react with sodium sulphate, barium sulphate and sodium chloride are formed. Give the equation for the. For each element, we check if the.
science chemistry precipitation reaction barium sulfate Fundamental
For each element, we check if the. When solutions of barium chloride (aq) and sodium sulfate (aq) are mixed, what is/are the spectator ion (s)? When barium chloride is made to react with sodium sulphate, barium sulphate and sodium chloride are formed. Sodium sulphate chemically reacts with barium chloride in the form of their aqueous solution to form a white.
Sodium Sulphate Chemically Reacts With Barium Chloride In The Form Of Their Aqueous Solution To Form A White Precipitate Of Barium.
Give the equation for the. Let's balance this equation using the inspection method. When barium chloride is made to react with sodium sulphate, barium sulphate and sodium chloride are formed. When solutions of barium chloride (aq) and sodium sulfate (aq) are mixed, what is/are the spectator ion (s)?